TLRs: Innate Immune Sentries against SARS-CoV-2 Infection
- PMID: 37175768
- PMCID: PMC10178469
- DOI: 10.3390/ijms24098065
TLRs: Innate Immune Sentries against SARS-CoV-2 Infection
Abstract
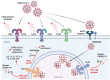
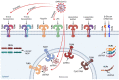
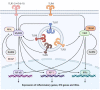
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been responsible for a devastating pandemic since March 2020. Toll-like receptors (TLRs), crucial components in the initiation of innate immune responses to different pathogens, trigger the downstream production of pro-inflammatory cytokines, interferons, and other mediators. It has been demonstrated that they contribute to the dysregulated immune response observed in patients with severe COVID-19. TLR2, TLR3, TLR4 and TLR7 have been associated with COVID-19 severity. Here, we review the role of TLRs in the etiology and pathogenesis of COVID-19, including TLR7 and TLR3 rare variants, the L412F polymorphism in TLR3 that negatively regulates anti-SARS-CoV-2 immune responses, the TLR3-related cellular senescence, the interaction of TLR2 and TLR4 with SARS-CoV-2 proteins and implication of TLR2 in NET formation by SARS-CoV-2. The activation of TLRs contributes to viral clearance and disease resolution. However, TLRs may represent a double-edged sword which may elicit dysregulated immune signaling, leading to the production of proinflammatory mediators, resulting in severe disease. TLR-dependent excessive inflammation and TLR-dependent antiviral response may tip the balance towards the former or the latter, altering the equilibrium that drives the severity of disease.
Keywords: COVID-19; SARS-CoV-2; Toll-like receptor; host genetics; innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Fallerini C., Picchiotti N., Baldassarri M., Zguro K., Daga S., Fava F., Benetti E., Amitrano S., Bruttini M., Palmieri M., et al. Common, low-frequency, rare, and ultra-rare coding variants contribute to COVID-19 severity. Hum. Genet. 2022;141:147–173. doi: 10.1007/s00439-021-02397-7. - DOI - PMC - PubMed
-
- Picchiotti N., Benetti E., Fallerini C., Daga S., Baldassarri M., Fava F., Zguro K., Valentino F., Doddato G., Giliberti A., et al. Post-Mendelian Genetic Model in COVID-19. Cardiol. Cardiovasc. Med. 2021;5:673–694. doi: 10.26502/fccm.92920232. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

