Phafins Are More Than Phosphoinositide-Binding Proteins
- PMID: 37175801
- PMCID: PMC10178739
- DOI: 10.3390/ijms24098096
Phafins Are More Than Phosphoinositide-Binding Proteins
Abstract
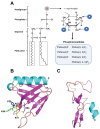
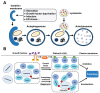
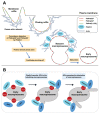
Phafins are PH (Pleckstrin Homology) and FYVE (Fab1, YOTB, Vac1, and EEA1) domain-containing proteins. The Phafin protein family is classified into two groups based on their sequence homology and functional similarity: Phafin1 and Phafin2. This protein family is unique because both the PH and FYVE domains bind to phosphatidylinositol 3-phosphate [PtdIns(3)P], a phosphoinositide primarily found in endosomal and lysosomal membranes. Phafin proteins act as PtdIns(3)P effectors in apoptosis, endocytic cargo trafficking, and autophagy. Additionally, Phafin2 is recruited to macropinocytic compartments through coincidence detection of PtdIns(3)P and PtdIns(4)P. Membrane-associated Phafins serve as adaptor proteins that recruit other binding partners. In addition to the phosphoinositide-binding domains, Phafin proteins present a poly aspartic acid motif that regulates membrane binding specificity. In this review, we summarize the involvement of Phafins in several cellular pathways and their potential physiological functions while highlighting the similarities and differences between Phafin1 and Phafin2. Besides, we discuss research perspectives for Phafins.
Keywords: FYVE domain; PH domain; Phafin; PtdIns(3)P; PtdIns(4)P; autoinhibition; membrane remodeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

