Polylevolysine and Fibronectin-Loaded Nano-Hydroxyapatite/PGLA/Dextran-Based Scaffolds for Improving Bone Regeneration: A Histomorphometric in Animal Study
- PMID: 37175849
- PMCID: PMC10179305
- DOI: 10.3390/ijms24098137
Polylevolysine and Fibronectin-Loaded Nano-Hydroxyapatite/PGLA/Dextran-Based Scaffolds for Improving Bone Regeneration: A Histomorphometric in Animal Study
Abstract
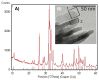
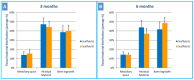
The regeneration of large bone defects is still demanding, requiring biocompatible scaffolds, with osteoconductive and osteoinductive properties. This study aimed to assess the pre-clinical efficacy of a nano-hydroxyapatite (nano-HA)/PGLA/dextran-based scaffold loaded with Polylevolysine (PLL) and fibronectin (FN), intended for bone regeneration of a critical-size tibial defect, using an ovine model. After physicochemical characterization, the scaffolds were implanted in vivo, producing two monocortical defects on both tibiae of ten adult sheep, randomly divided into two groups to be euthanized at three and six months after surgery. The proximal left and right defects were filled, respectively, with the test scaffold (nano-HA/PGLA/dextran-based scaffold loaded with PLL and FN) and the control scaffold (nano-HA/PGLA/dextran-based scaffold not loaded with PLL and FN); the distal defects were considered negative control sites, not receiving any scaffold. Histological and histomorphometric analyses were performed to quantify the bone ingrowth and residual material 3 and 6 months after surgery. In both scaffolds, the morphological analyses, at the SEM, revealed the presence of submicrometric crystals on the surfaces and within the scaffolds, while optical microscopy showed a macroscopic 3D porous architecture. XRD confirmed the presence of nano-HA with a high level of crystallinity degree. At the histological and histomorphometric evaluation, new bone formation and residual biomaterial were detectable inside the defects 3 months after intervention, without differences between the scaffolds. At 6 months, the regenerated bone was significantly higher in the defects filled with the test scaffold (loaded with PLL and FN) than in those filled with the control scaffold, while the residual material was higher in correspondence to the control scaffold. Nano-HA/PGLA/dextran-based scaffolds loaded with PLL and FN appear promising in promoting bone regeneration in critical-size defects, showing balanced regenerative and resorbable properties to support new bone deposition.
Keywords: Polylevolysine; bone regeneration; critical-size bone defect; fibronectin; hydroxyapatite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Kengelbach-Weigand A., Thielen C., Bäuerle T., Götzl R., Gerber T., Körner C., Beier J.P., Horch R.E., Boos A.M. Personalized Medicine for Reconstruction of Critical-Size Bone Defects—A Translational Approach with Customizable Vascularized Bone Tissue. Npj Regen. Med. 2021;6:49. doi: 10.1038/s41536-021-00158-8. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

