Multi-Targeting Neuroprotective Effects of Syzygium aromaticum Bud Extracts and Their Key Phytocompounds against Neurodegenerative Diseases
- PMID: 37175851
- PMCID: PMC10178913
- DOI: 10.3390/ijms24098148
Multi-Targeting Neuroprotective Effects of Syzygium aromaticum Bud Extracts and Their Key Phytocompounds against Neurodegenerative Diseases
Abstract
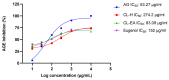
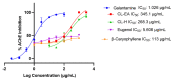
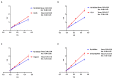
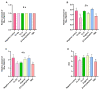
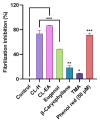
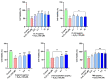
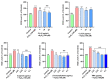
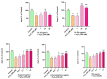
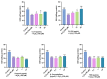
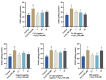
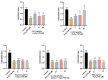
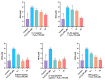
Alzheimer's disease (AD) is a neurodegenerative disease that causes a gradual loss of normal motor and cognitive function. The complex AD pathophysiology involves various factors such as oxidative stress, neuroinflammation, amyloid-beta (Aβ) aggregation, disturbed neurotransmission, and apoptosis. The available drugs suffer from a range of side effects and are not able to cover different aspects of the disease. Therefore, finding a safer therapeutic approach that can affect multiple targets at a time is highly desirable. In the present study, the underlying neuroprotective mechanism of an important culinary spice, Syzygium aromaticum (Clove) extract, and major bioactive compounds were studied in hydrogen peroxide-induced oxidative stress in human neuroblastoma SH-SY5Y cell lines as a model. The extracts were subjected to GC-MS to identify important bioactive components. The extracts and key bio-actives reduced reactive oxygen species (ROS), restored mitochondrial membrane potential (MMP), and provided neuroprotection from H2O2-induced oxidative stress in cell-based assays due to the antioxidant action. They also reduced lipid peroxidation significantly and restored GSH content. Clove extracts have also displayed anti-acetylcholinesterase (AChE) activity, anti-glycation potential, and Aβ aggregation/fibrilization inhibition. The multitarget neuroprotective approach displayed by Clove makes it a potential candidate for AD drug development.
Keywords: Alzheimer’s disease; H2O2 induced stress; MDS; Syzygium aromaticum; anti-acetylcholine esterase activity; anti-glycation; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

