A Tumor and Immune-Related Micro-RNA Signature Predicts Relapse-Free Survival of Melanoma Patients Treated with Ipilimumab
- PMID: 37175874
- PMCID: PMC10179521
- DOI: 10.3390/ijms24098167
A Tumor and Immune-Related Micro-RNA Signature Predicts Relapse-Free Survival of Melanoma Patients Treated with Ipilimumab
Abstract
Despite the unprecedented advances in the treatment of melanoma with immunotherapy, there continues to be a major need for biomarkers of clinical benefits and immune resistance associated with immune checkpoint inhibitors; microRNA could play a vital role in these efforts. This study planned to identify differentially expressed miRNA molecules that may have prognostic value for clinical benefits. Patients with surgically operable regionally advanced melanoma were treated with neoadjuvant ipilimumab (10 mg/kg intravenously every 3 weeks × two doses) bracketing surgery. Tumor biospecimens were obtained at baseline and surgery, and microRNA (miRNA) expression profiling was performed on the tumor biopsies. We found that an expression profile consisting of a 4-miRNA signature was significantly associated with improved relapse-free survival (RFS). The signature consisted of biologically relevant molecules previously reported to have prognostic value in melanoma and other malignancies, including miR-34c, miR-711, miR-641, and miR-22. Functional annotation analysis of target genes for the 4-miRNA signature was significantly enriched for various cancer-related pathways, including cell proliferation regulation, apoptosis, the MAPK signaling pathway, and the positive regulation of T cell activation. Our results presented miRNAs as potential biomarkers that can guide the treatment of melanoma with immune checkpoint inhibitors. These findings warrant further investigation in relation to CTLA4 blockade and other immune checkpoint inhibitors. ClinicalTrials.gov NCT00972933.
Keywords: biomarkers; immunotherapy; melanoma; miRNA; microRNA.
Conflict of interest statement
Iyad Kobeissi, Islam Eljilany, Tala Achkar, William A. LaFramboise, Lucas Santana-Santos declare that they have no conflict of interest. Ahmad A. Tarhini declares contracted research grants with institution from Bristol Myers Squib, Genentech-Roche, Regeneron, Sanofi-Genzyme, Nektar, Clinigen, Merck, Acrotech, Pfizer, Checkmate, OncoSec, Scholar Rock, InflaRx GmbH, Agenus; personal consultant/advisory board fees (less than $10k per year) from Bristol Myers Squibb, Merck, Easai, Instil Bio, Clinigin, Regeneron, Sanofi-Genzyme, Novartis, Partner Therapeutics, Genentech/Roche, BioNTech, Concert AI, AstraZeneca outside the submitted work.
Figures
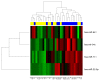
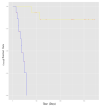

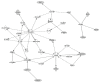
References
-
- American Cancer Society . Cancer Facts & Figures 2023. American Cancer Society; Atlanta, GA, USA: 2023.
-
- Gershenwald J.E., Scolyer R.A., Hess K.R., Sondak V.K., Long G.V., Ross M.I., Lazar A.J., Faries M.B., Kirkwood J.M., McArthur G.A., et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017;67:472–492. doi: 10.3322/caac.21409. - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

