A Split-Marker System for CRISPR-Cas9 Genome Editing in Methylotrophic Yeasts
- PMID: 37175878
- PMCID: PMC10179152
- DOI: 10.3390/ijms24098173
A Split-Marker System for CRISPR-Cas9 Genome Editing in Methylotrophic Yeasts
Abstract
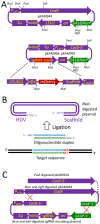
Methylotrophic yeasts such as Ogataea polymorpha and Komagataella phaffii (sin. Hansenula polymorpha and Pichia pastoris, respectively) are commonly used in basic research and biotechnological applications, frequently those requiring genome modifications. However, the CRISPR-Cas9 genome editing approaches reported for these species so far are relatively complex and laborious. In this work we present an improved plasmid vector set for CRISPR-Cas9 genome editing in methylotrophic yeasts. This includes a plasmid encoding Cas9 with a nuclear localization signal and plasmids with a scaffold for the single guide RNA (sgRNA). Construction of a sgRNA gene for a particular target sequence requires only the insertion of a 24 bp oligonucleotide duplex into the scaffold. Prior to yeast transformation, each plasmid is cleaved at two sites, one of which is located within the selectable marker, so that the functional marker can be restored only via recombination of the Cas9-containing fragment with the sgRNA gene-containing fragment. This recombination leads to the formation of an autonomously replicating plasmid, which can be lost from yeast clones after acquisition of the required genome modification. The vector set allows the use of G418-resistance and LEU2 auxotrophic selectable markers. The functionality of this setup has been demonstrated in O. polymorpha, O. parapolymorpha, O. haglerorum and Komagataella phaffii.
Keywords: CRISPR; Cas9; Komagataella; Ogataea; genome editing; genome engineering; methylotrophic yeast.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Supplementary concepts
Grants and funding
- 075-15-2021-1071/The Ministry of Science and Higher Education of the Russian Federation, grant for the Development of genomic editing technologies for innovation in industrial biotechnology
- 075-15-2019-1659/The Ministry of Science and Higher Education of the Russian Federation, grant for the Development of genomic editing technologies for innovation in industrial biotechnology
- 20-14-00286/Russian Science Foundation
- base funding/The Ministry of Science and Higher Education of the Russian Federation
LinkOut - more resources
Full Text Sources
Research Materials

