Novel Susceptibility Genes Drive Familial Non-Medullary Thyroid Cancer in a Large Consanguineous Kindred
- PMID: 37175943
- PMCID: PMC10179265
- DOI: 10.3390/ijms24098233
Novel Susceptibility Genes Drive Familial Non-Medullary Thyroid Cancer in a Large Consanguineous Kindred
Abstract
Familial non-medullary thyroid cancer (FNMTC) is a well-differentiated thyroid cancer (DTC) of follicular cell origin in two or more first-degree relatives. Patients typically demonstrate an autosomal dominant inheritance pattern with incomplete penetrance. While known genes and chromosomal loci account for some FNMTC, the molecular basis for most FNMTC remains elusive. To identify the variation(s) causing FNMTC in an extended consanguineous family consisting of 16 papillary thyroid carcinoma (PTC) cases, we performed whole exome sequence (WES) analysis of six family patients. We demonstrated an association of ARHGEF28, FBXW10, and SLC47A1 genes with FNMTC. The variations in these genes may affect the structures of their encoded proteins and, thus, their function. The most promising causative gene is ARHGEF28, which has high expression in the thyroid, and its protein-protein interactions (PPIs) suggest predisposition of PTC through ARHGEF28-SQSTM1-TP53 or ARHGEF28-PTCSC2-FOXE1-TP53 associations. Using DNA from a patient's thyroid malignant tissue, we analyzed the possible cooperation of somatic variations with these genes. We revealed two somatic heterozygote variations in XRCC1 and HRAS genes known to implicate thyroid cancer. Thus, the predisposition by the germline variations and a second hit by somatic variations could lead to the progression to PTC.
Keywords: ARHGEF28; FBXW10; HRAS; SLC47A1; XRCC1; familial non-medullary thyroid cancer (FNMTC); papillary thyroid carcinoma (PTC); protein–protein interactions (PPIs); whole exome sequence (WES).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
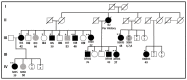
 : top—patient’s code; bottom—patient’s age at diagnosis.
: top—patient’s code; bottom—patient’s age at diagnosis.  : number of individuals.
: number of individuals.
References
-
- Shaha A.R., Migliacci J.C., Nixon I., Wang L.Y., Wong R.J., Morris L.G., Patel S.G., Shah J.P., Tuttle R.M., Ganly I. Stage migration with the new American Joint Committee on Cancer (AJCC) staging system (8th edition) for differentiated thyroid cancer. Surgery. 2019;165:6–11. doi: 10.1016/j.surg.2018.04.078. - DOI - PMC - PubMed
-
- Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

