In Vivo Radiobiological Investigations with the TOP-IMPLART Proton Beam on a Medulloblastoma Mouse Model
- PMID: 37175984
- PMCID: PMC10179102
- DOI: 10.3390/ijms24098281
In Vivo Radiobiological Investigations with the TOP-IMPLART Proton Beam on a Medulloblastoma Mouse Model
Abstract
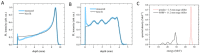
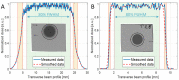
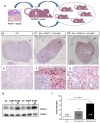
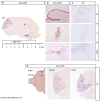
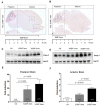
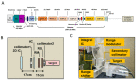
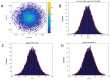
Protons are now increasingly used to treat pediatric medulloblastoma (MB) patients. We designed and characterized a setup to deliver proton beams for in vivo radiobiology experiments at a TOP-IMPLART facility, a prototype of a proton-therapy linear accelerator developed at the ENEA Frascati Research Center, with the goal of assessing the feasibility of TOP-IMPLART for small animal proton therapy research. Mice bearing Sonic-Hedgehog (Shh)-dependent MB in the flank were irradiated with protons to test whether irradiation could be restricted to a specific depth in the tumor tissue and to compare apoptosis induced by the same dose of protons or photons. In addition, the brains of neonatal mice at postnatal day 5 (P5), representing a very small target, were irradiated with 6 Gy of protons with two different collimated Spread-Out Bragg Peaks (SOBPs). Apoptosis was visualized by immunohistochemistry for the apoptotic marker caspase-3-activated, and quantified by Western blot. Our findings proved that protons could be delivered to the upper part while sparing the deepest part of MB. In addition, a comparison of the effectiveness of protons and photons revealed a very similar increase in the expression of cleaved caspase-3. Finally, by using a very small target, the brain of P5-neonatal mice, we demonstrated that the proton irradiation field reached the desired depth in brain tissue. Using the TOP-IMPLART accelerator we established setup and procedures for proton irradiation, suitable for translational preclinical studies. This is the first example of in vivo experiments performed with a "full-linac" proton-therapy accelerator.
Keywords: apoptosis; cerebellum; dosimetry; linac; medulloblastoma; preclinical mouse models; proton therapy; small-field irradiation.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Peris-Bonet R., Martínez-García C., Lacour B., Petrovich S., Giner-Ripoll B., Navajas A., Steliarova-Foucher E. Childhood central nervous system tumours—Incidence and survival in Europe (1978–1997): Report from Automated Childhood Cancer Information System project. Eur. J. Cancer. 2006;42:2064–2080. doi: 10.1016/j.ejca.2006.05.009. - DOI - PubMed
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. - DOI - PubMed
-
- Kool M., Korshunov A., Remke M., Jones D.T., Schlanstein M., Northcott P.A., Cho Y.J., Koster J., Schouten-van Meeteren A., van Vuurden D., et al. Molecular subgroups of medulloblastoma: An international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–484. doi: 10.1007/s00401-012-0958-8. - DOI - PMC - PubMed
-
- Packer R.J., Sutton L.S., Elterman R., Lange B., Goldwein J., Nicholson S., Mulne L., Boyett J., D’angio G., Wechsler-jentzsch K., et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. Neurosurg. 1994;81:690–698. doi: 10.3171/jns.1994.81.5.0690. - DOI - PubMed
-
- Eaton B.R., Fong G.W., Ingerski L.M., Pulsifer M.B., Goyal S., Zhang C., Weyman E.A., Esiashvili N., Klosky J.L., MacDonald T.J., et al. Intellectual functioning among case-matched cohorts of children treated with proton or photon radiation for standard-risk medulloblastoma. Cancer. 2021;127:3840–3846. doi: 10.1002/cncr.33774. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

