Evaluation of the Different Nutritional and Environmental Parameters on Microbial Pyrene Degradation by Mangrove Culturable Bacteria
- PMID: 37175988
- PMCID: PMC10179275
- DOI: 10.3390/ijms24098282
Evaluation of the Different Nutritional and Environmental Parameters on Microbial Pyrene Degradation by Mangrove Culturable Bacteria
Abstract
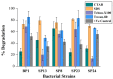
Mangrove ecosystems play curial roles in providing many ecological services and alleviating global climate change. However, they are in decline globally, mainly threatened by human activities and global warming, and organic pollutants, especially PAHs, are among the crucial reasons. Microbial remediation is a cost-effective and environmentally friendly way of alleviating PAH contamination. Therefore, understanding the effects of environmental and nutritional parameters on the biodegradation of polycyclic aromatic hydrocarbons (PAHs) is significant for the bioremediation of PAH contamination. In the present study, five bacterial strains, designated as Bp1 (Genus Rhodococcus), Sp8 (Genus Nitratireductor), Sp13 (Genus Marinobacter), Sp23 (Genus Pseudonocardia), and Sp24 (Genus Mycolicibacterium), have been isolated from mangrove sediment and their ring hydroxylating dioxygenase (RHD) genes have been successfully amplified. Afterward, their degradation abilities were comprehensively evaluated under normal cultural (monoculture and co-culture) and different nutritional (tryptone, yeast extract, peptone, glucose, sucrose, and NPK fertilizer) and environmental (cetyl trimethyl ammonium bromide (CTAB), sodium dodecyl sulfate (SDS)) parameters, as well with different co-contaminants (phenanthrene and naphthalene) and heavy metals (Cd2+, Cu2+, Fe3+, Ni2+, Mg2+, Mn2+, and Co2+). The results showed that strain Sp24 had the highest pyrene degradation rate (85%) in the monoculture experiment after being cultured for 15 days. Adding nitrogen- and carbon-rich sources, including tryptone, peptone, and yeast extract, generally endorsed pyrene degradation. In contrast, the effects of carbon sources (glucose and sucrose) on pyrene degradation were distinct for different bacterial strains. Furthermore, the addition of NPK fertilizer, SDS, Tween-80, phenanthrene, and naphthalene enhanced the bacterial abilities of pyrene removal significantly (p < 0.05). Heavy metals significantly reduced all bacterial isolates' degradation potentials (p < 0.05). The bacterial consortia containing high bio-surfactant-producing strains showed substantially higher pyrene degradation. Moreover, the consortia of three and five bacterial strains showed more degradation efficiency than those of two bacterial strains. These results provide helpful microbial resources for mangrove ecological remediation and insight into optimized culture strategies for the microbial degradation of PAHs.
Keywords: co-contamination; co-culture; degradation; heavy metals; nutrient supplements; pyrene.
Conflict of interest statement
The authors declared no conflict of interest.
Figures







References
-
- Apostolopoulou M.V., Monteyne E., Krikonis K., Pavlopoulos K., Roose P., Dehairs F. Monitoring polycyclic aromatic hydrocarbons in the Northeast Aegean Sea using Posidonia oceanica seagrass and synthetic passive samplers. Mar. Pollut. Bull. 2014;87:338–344. doi: 10.1016/j.marpolbul.2014.07.051. - DOI - PubMed
-
- Goswami P., Ohura T., Guruge K.S., Yoshioka M., Yamanaka N., Akiba M., Munuswamy N. Spatio-temporal distribution, source, and genotoxic potential of polycyclic aromatic hydrocarbons in estuarine and riverine sediments from southern India. Ecotoxicol. Environ. Saf. 2016;130:113–123. doi: 10.1016/j.ecoenv.2016.04.016. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2022YFC3102003; 2022YFC3102004; 2021YFC3100500/National Key Research and Development Program of China
- 422QN440/Hainan Provincial Natural Science Foundation of China
- 41676163, 42276160/National Natural Science Foundation of China
- ISEE2021ZD03/Innovation Academy of South China Sea Ecology and Environmental Engineering, Chinese Academy of Sciences
- 2020B1212060058; 2021B1212050023/Science and Technology Planning Project of Guangdong Province, China
LinkOut - more resources
Full Text Sources

