Prospects and Advances in Adoptive Natural Killer Cell Therapy for Unmet Therapeutic Needs in Pediatric Bone Sarcomas
- PMID: 37176035
- PMCID: PMC10178897
- DOI: 10.3390/ijms24098324
Prospects and Advances in Adoptive Natural Killer Cell Therapy for Unmet Therapeutic Needs in Pediatric Bone Sarcomas
Abstract
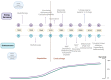
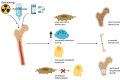
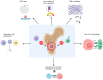
Malignant bone tumors are aggressive tumors, with a high tendency to metastasize, that are observed most frequently in adolescents during rapid growth spurts. Pediatric patients with malignant bone sarcomas, Ewing sarcoma and osteosarcoma, who present with progressive disease have dire survival rates despite aggressive therapy. These therapies can have long-term effects on bone growth, such as decreased bone mineral density and reduced longitudinal growth. New therapeutic approaches are therefore urgently needed for targeting pediatric malignant bone tumors. Harnessing the power of the immune system against cancer has improved the survival rates dramatically in certain cancer types. Natural killer (NK) cells are a heterogeneous group of innate effector cells that possess numerous antitumor effects, such as cytolysis and cytokine production. Pediatric sarcoma cells have been shown to be especially susceptible to NK-cell-mediated killing. NK-cell adoptive therapy confers numerous advantages over T-cell adoptive therapy, including a good safety profile and a lack of major histocompatibility complex restriction. NK-cell immunotherapy has the potential to be a new therapy for pediatric malignant bone tumors. In this manuscript, we review the general characteristics of osteosarcoma and Ewing sarcoma, discuss the long-term effects of sarcoma treatment on bones, and the barriers to effective immunotherapy in bone sarcomas. We then present the laboratory and clinical studies on NK-cell immunotherapy for pediatric malignant bone tumors. We discuss the various donor sources and NK-cell types, the engineering of NK cells and combinatorial treatment approaches that are being studied to overcome the current challenges in adoptive NK-cell therapy, while suggesting approaches for future studies on NK-cell immunotherapy in pediatric bone tumors.
Keywords: Ewing sarcoma; NK cells; adoptive cell therapy; immune evasion; immunotherapy; osteosarcoma; pediatric bone sarcoma; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Steliarova-Foucher E., Colombet M., Ries L.A.G., Moreno F., Dolya A., Bray F., Hesseling P., Shin H.Y., Stiller C.A., IICC-3 Contributors et al. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017;18:719–731. doi: 10.1016/S1470-2045(17)30186-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

