Ubiquitination Links DNA Damage and Repair Signaling to Cancer Metabolism
- PMID: 37176148
- PMCID: PMC10179089
- DOI: 10.3390/ijms24098441
Ubiquitination Links DNA Damage and Repair Signaling to Cancer Metabolism
Abstract
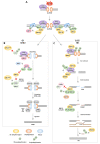
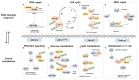
Changes in the DNA damage response (DDR) and cellular metabolism are two important factors that allow cancer cells to proliferate. DDR is a set of events in which DNA damage is recognized, DNA repair factors are recruited to the site of damage, the lesion is repaired, and cellular responses associated with the damage are processed. In cancer, DDR is commonly dysregulated, and the enzymes associated with DDR are prone to changes in ubiquitination. Additionally, cellular metabolism, especially glycolysis, is upregulated in cancer cells, and enzymes in this metabolic pathway are modulated by ubiquitination. The ubiquitin-proteasome system (UPS), particularly E3 ligases, act as a bridge between cellular metabolism and DDR since they regulate the enzymes associated with the two processes. Hence, the E3 ligases with high substrate specificity are considered potential therapeutic targets for treating cancer. A number of small molecule inhibitors designed to target different components of the UPS have been developed, and several have been tested in clinical trials for human use. In this review, we discuss the role of ubiquitination on overall cellular metabolism and DDR and confirm the link between them through the E3 ligases NEDD4, APC/CCDH1, FBXW7, and Pellino1. In addition, we present an overview of the clinically important small molecule inhibitors and implications for their practical use.
Keywords: DNA damage response; DNA repair; E3 ligase; cancer metabolism; therapeutics; ubiquitination.
Conflict of interest statement
The authors declares no conflict of interest.
Figures
Similar articles
-
CRL4 Ubiquitin Pathway and DNA Damage Response.Adv Exp Med Biol. 2020;1217:225-239. doi: 10.1007/978-981-15-1025-0_14. Adv Exp Med Biol. 2020. PMID: 31898231 Review.
-
Ubiquitination and deubiquitination: Implications on cancer therapy.Biochim Biophys Acta Gene Regul Mech. 2023 Dec;1866(4):194979. doi: 10.1016/j.bbagrm.2023.194979. Epub 2023 Aug 24. Biochim Biophys Acta Gene Regul Mech. 2023. PMID: 37633647 Review.
-
The NEDD4 ubiquitin E3 ligase: a snapshot view of its functional activity and regulation.Biochem Soc Trans. 2022 Feb 28;50(1):473-485. doi: 10.1042/BST20210731. Biochem Soc Trans. 2022. PMID: 35129615 Review.
-
A20/TNFAIP3 Regulates the DNA Damage Response and Mediates Tumor Cell Resistance to DNA-Damaging Therapy.Cancer Res. 2018 Feb 15;78(4):1069-1082. doi: 10.1158/0008-5472.CAN-17-2143. Epub 2017 Dec 12. Cancer Res. 2018. PMID: 29233925
-
RNF168 promotes noncanonical K27 ubiquitination to signal DNA damage.Cell Rep. 2015 Jan 13;10(2):226-38. doi: 10.1016/j.celrep.2014.12.021. Epub 2015 Jan 8. Cell Rep. 2015. PMID: 25578731
Cited by
-
Ubiquitination and deubiquitination in cancer: from mechanisms to novel therapeutic approaches.Mol Cancer. 2024 Jul 25;23(1):148. doi: 10.1186/s12943-024-02046-3. Mol Cancer. 2024. PMID: 39048965 Free PMC article. Review.
-
Prognostic and Immune Landscape Analysis of Ubiquitination-related Genes in Hepatocellular Carcinoma: Based on Bulk and Single-cell RNA Sequencing Data.J Cancer. 2024 Mar 11;15(9):2580-2600. doi: 10.7150/jca.93425. eCollection 2024. J Cancer. 2024. PMID: 38577593 Free PMC article.
-
Crosstalk between O-GlcNAcylation and phosphorylation in metabolism: regulation and mechanism.Cell Death Differ. 2025 Jul;32(7):1181-1199. doi: 10.1038/s41418-025-01473-z. Epub 2025 Mar 5. Cell Death Differ. 2025. PMID: 40045035 Review.
-
Transcriptional regulation and post-translational modifications in the glycolytic pathway for targeted cancer therapy.Acta Pharmacol Sin. 2024 Aug;45(8):1533-1555. doi: 10.1038/s41401-024-01264-1. Epub 2024 Apr 15. Acta Pharmacol Sin. 2024. PMID: 38622288 Free PMC article. Review.
-
O‑GlcNAcylation: The crosstalk between infection immunity and autophagy in sepsis (Review).Mol Med Rep. 2025 Oct;32(4):281. doi: 10.3892/mmr.2025.13646. Epub 2025 Aug 8. Mol Med Rep. 2025. PMID: 40776755 Free PMC article. Review.
References
-
- Lilley C.E., Chaurushiya M.S., Boutell C., Landry S., Suh J., Panier S., Everett R.D., Stewart G.S., Durocher D., Weitzman M.D. A viral E3 ligase targets RNF8 and RNF168 to control histone ubiquitination and DNA damage responses. EMBO J. 2010;29:943–955. doi: 10.1038/emboj.2009.400. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

