Synthetic Calcium Silicate Biocomposite Based on Sea Urchin Skeleton for 5-Fluorouracil Cancer Delivery
- PMID: 37176377
- PMCID: PMC10180529
- DOI: 10.3390/ma16093495
Synthetic Calcium Silicate Biocomposite Based on Sea Urchin Skeleton for 5-Fluorouracil Cancer Delivery
Abstract
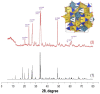
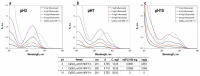
Synthetic calcium silicates and phosphates are promising compounds for targeted drug delivery for the effective treatment of cancerous tumors, and for minimizing toxic effects on the patient's entire body. This work presents an original synthesis of a composite based on crystalline wollastonite CaSiO3 and combeite Na4Ca4(Si6O18), using a sea urchin Mesocentrotus nudus skeleton by microwave heating under hydrothermal conditions. The phase and elemental composition and structure of the obtained composite were studied by XRF, REM, BET, and EDS methods, depending on the microwave heating time of 30 or 60 min, respectively, and the influence of thermo-oxidative post-treatment of samples. The role of the sea urchin skeleton in the synthesis was shown. First, it provides a raw material base (source of Ca2+) for the formation of the calcium silicate composite. Second, it is a matrix for the formation of its porous inorganic framework. The sorption capacity of the composite, with respect to 5-fluorouracil, was estimated, the value of which was 12.3 mg/L. The resulting composite is a promising carrier for the targeted delivery of chemotherapeutic drugs. The mechanism of drug release from an inorganic natural matrix was also evaluated by fitting its release profile to various mathematical models.
Keywords: calcium silicate; drug delivery system; hydrothermal synthesis; microwave synthesis; sea urchin skeleton; wollastonite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

