Response to Hypersalinity of Four Halophytes Growing in Hydroponic Floating Systems: Prospects in the Phytomanagement of High Saline Wastewaters and Extreme Environments
- PMID: 37176795
- PMCID: PMC10181242
- DOI: 10.3390/plants12091737
Response to Hypersalinity of Four Halophytes Growing in Hydroponic Floating Systems: Prospects in the Phytomanagement of High Saline Wastewaters and Extreme Environments
Abstract
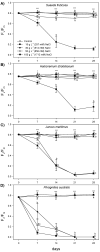
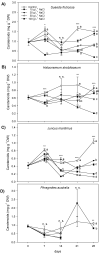
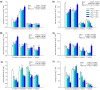
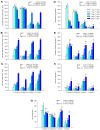
Hypersaline environments occur naturally worldwide in arid and semiarid regions or in artificial areas where the discharge of highly saline wastewaters, such as produced water (PW) from oil and gas industrial setups, has concentrated salt (NaCl). Halophytes can tolerate high NaCl concentrations by adopting ion extrusion and inclusion mechanisms at cell, tissue, and organ levels; however, there is still much that is not clear in the response of these plants to salinity and completely unknown issues in hypersaline conditions. Mechanisms of tolerance to saline and hypersaline conditions of four different halophytes (Suaeda fruticosa (L.) Forssk, Halocnemum strobilaceum (Pall.) M. Bieb., Juncus maritimus Lam. and Phragmites australis (Cav.) Trin. ex Steudel) were assessed by analysing growth, chlorophyll fluorescence and photosynthetic pigment parameters, nutrients, and sodium (Na) uptake and distribution in different organs. Plants were exposed to high saline (257 mM or 15 g L-1 NaCl) and extremely high or hypersaline (514, 856, and 1712 mM or 30, 50, and 100 g L-1 NaCl) salt concentrations in a hydroponic floating culture system for 28 days. The two dicotyledonous S. fruticosa and H. strobilaceum resulted in greater tolerance to hypersaline concentrations than the two monocotyledonous species J. maritimus and P. australis. Plant biomass and major cation (K, Ca, and Mg) distributions among above- and below-ground organs evidenced the osmoprotectant roles of K in the leaves of S. fruticosa, and of Ca and Mg in the leaves and stem of H. strobilaceum. In J. maritimus and P. australis the rhizome modulated the reduced uptake and translocation of nutrients and Na to shoot with increasing salinity levels. S. fruticosa and H. strobilaceum absorbed and accumulated elevated Na amounts in the aerial parts at all the NaCl doses tested, with high bioaccumulation (from 0.5 to 8.3) and translocation (1.7-16.2) factors. In the two monocotyledons, Na increased in the root and rhizome with the increasing concentration of external NaCl, dramatically reducing the growth in J. maritimus at both 50 and 100 g L-1 NaCl and compromising the survival of P. australis at 30 g L-1 NaCl and over after two weeks of treatment.
Keywords: fluorescence parameters; hypersaline environment; nutrient absorption; organ element distribution; photosynthetic pigments; phytodesalinization; sodium content.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Tweedley J.R., Dittmann S.R., Whitfield A.K., Withers K., Hoeksema S.D., Potter I.C. Hypersalinity: Global distribution, causes, and present and future effects on the biota of estuaries and lagoons. In: Wolanski E., Day J.W., Elliott M., Ramachandran R., editors. Coasts and Estuaries: The Future. Elsevier; Amsterdam, The Netherlands: 2019. pp. 523–546. - DOI
-
- Zaman M., Shahid S.A., Heng L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques, Joint FAO/IAEA Programme Nuclear Techniques in Food and Agriculture. Springer Nature Switzerland AG; Cham, Switzerland: 2018. - DOI
-
- FAO Food and Agriculture: Key to Achieving the 2030, Agenda for Sustainable Development. [(accessed on 19 December 2022)]. Job No. I5499, Food and Agriculture Organization of the United Nations 2016, Rome, 23. Available online: https://www.fao.org/3/i5499e/i5499e.pdf.
Grants and funding
LinkOut - more resources
Full Text Sources

