Development of In Vitro Anther Culture for Doubled Haploid Plant Production in Indica Rice (Oryza sativa L.) Genotypes
- PMID: 37176830
- PMCID: PMC10180916
- DOI: 10.3390/plants12091774
Development of In Vitro Anther Culture for Doubled Haploid Plant Production in Indica Rice (Oryza sativa L.) Genotypes
Abstract
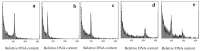
Anther culture is an efficient biotechnological tool in modern plant breeding programs to produce new varieties and parental lines in hybrid seed productions. However, some bottlenecks-low induction rate, genotype dependency, albinism-restrict the widespread utilization of in vitro anther culture in rice breeding, especially in Oryza sativa ssp. indica (indica) genotypes, while an improved efficient protocol can shorten the process of breeding. Three different induction media (N6NDK, N6NDZ, Ali-1) and four plant regeneration media (mMSNBK1, MSNBK3, MSNBKZ1, MSNBKZ2) were tested with five indica rice genotypes to increase the efficiency of in vitro androgenesis (number of calli and regenerated green plantlets). The production of calli was more efficient on the N6NDK medium with an average 88.26 calli/100 anthers and N6NDZ medium with an average of 103.88 calli/100 anthers as compared to Ali-1 with an average of 6.96 calli/100 anthers. The production of green plantlets was greater when calli was produced on N6NDK medium (2.15 green plantlets/100 anthers) compared when produced on to N6NDZ medium (1.18 green plantlets/100 anthers). Highest green plantlets production (4.7 green plantlets/100 anthers) was achieved when mMSNBK1 plant regeneration medium was used on calli produced utilizing N6NDK induction medium. In the best overall treatment (N6NDK induction medium and mMSNBK1 plant regeneration medium), four tested genotypes produced green plantlets. However, the genotype influenced the efficiency, and the green plantlets production ranged from 0.4 green plantlets/100 anthers to 8.4 green plantlets/100 anthers. The ploidy level of 106 acclimatized indica rice plantlets were characterized with flow cytometric analyses to calculate the percentage of spontaneous chromosome doubling. Altogether, 48 haploid-, 55 diploid-, 2 tetraploid- and 1 mixoploid plantlets were identified among the regenerant plantlets, and the spontaneous chromosome doubling percentage was 51.89%. Utilization of DH plants have been integrated as a routine method in the Hungarian rice breeding program. The tetraploid lines can be explored for their potential to offer new scopes for rice research and breeding directions in the future.
Keywords: Oryza sativa L.; androgenesis; anther culture; flow cytometry; haploid; indica.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Thomas W.T.B., Forster B.P., Gertsson B. Doubled Haploids in Breeding. In: Maluszynski M., Kasha K.J., Forster B.P., Szarejko I., editors. Doubled Haploid Production in Crop Plants, a Manual. Kluwer Academic Publisher; Dordrecht, The Netherlands: 2003. pp. 337–349. - DOI
-
- Zapata-Arias F.J. Laboratory Protocol for Anther Culture Technique in Rice. In: Maluszynski M., Kasha K.J., Forster B.P., Szarejko I., editors. Doubled Haploid Production in Crop Plants, a Manual. Kluwer Academic Publisher; Dordrecht, The Netherlands: 2003. pp. 109–116. - DOI
-
- Pauk J., Jancsó M., Simon-Kiss I. Rice Doubled Haploids and Breeding. In: Touraev A., Foster B.P., Jain S.M., editors. Advances in Haploid Production in Higher Plants. Springer Science; Berlin/Heidelberg, Germany: 2009. pp. 189–197. - DOI
-
- Serrat X., Cardona M., Gil J., Brito A.M., Moysset L., Nogues S., Lalanne E. A Mediterranean japonica rice (Oryza sativa) cultivar improvement through anther culture. Euphytica. 2013;195:31–44. doi: 10.1007/s10681-013-0955-6. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

