An In Vitro and In Vivo Assessment of Antitumor Activity of Extracts Derived from Three Well-Known Plant Species
- PMID: 37176897
- PMCID: PMC10180766
- DOI: 10.3390/plants12091840
An In Vitro and In Vivo Assessment of Antitumor Activity of Extracts Derived from Three Well-Known Plant Species
Abstract
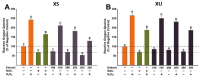
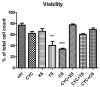
One of the objectives of this study consists of the assessment of the antitumor activity of several extracts from three selected plant species: Xanthium spinosum L., Trifolium pratense L., and Coffea arabica L. and also a comparative study of this biological activity, with the aim of establishing a superior herbal extract for antitumor benefits. The phytochemical profile of the extracts was established by HPLC-MS analysis. Further, the selected extracts were screened in vitro for their antitumor activity and antioxidant potential on two cancer cell lines: A549-human lung adenocarcinoma and T47D-KBluc-human breast carcinoma and on normal cells. One extract per plant was selected for in vivo assessment of antitumor activity in an Ehrlich ascites mouse model. The extracts presented high content of antitumor compounds such as caffeoylquinic acids in the case of X. spinosum L. (7.22 µg/mL-xanthatin, 4.611 µg/mL-4-O-caffeoylquinic acid) and green coffee beans (10.008 µg/mL-cafestol, 265.507 µg/mL-4-O-caffeoylquinic acid), as well as isoflavones in the case of T. pratense L. (6806.60 ng/mL-ononin, 102.78 µg/mL-biochanin A). Concerning the in vitro results, the X. spinosum L. extracts presented the strongest anticancerous and antioxidant effects. In vivo, ascites cell viability decreased after T. pratense L. and green coffee bean extracts administration, whereas the oxidative stress reduction potential was important in tumor samples after T. pratense L. Cell viability was also decreased after administration of cyclophosphamide associated with X. spinosum L. and T. pratense L. extracts, respectively. These results suggested that T. pratense L. or X. spinosum L. extracts in combination with chemotherapy can induce lipid peroxidation in tumor cells and decrease the tumor viability especially, T. pratense L. extract.
Keywords: Trifolium pratense; Xanthium spinosum; antioxidant activity; antitumor activity; caffeoylquinic acid; green coffee beans; isoflavones; xanthanolides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. - DOI - PubMed
-
- Chhikara B.S., Parang K. Global Cancer Statistics 2022: The trends projection analysis. [(accessed on 27 March 2023)];Chem. Biol. Lett. 2023 10:451. Available online: https://pubs.thesciencein.org/cbl.
-
- do Carmo M.A.V., Pressete C.G., Marques M.J., Granato D., Azevedo L. Polyphenols as potential antiproliferative agents: Scientific trends. Curr. Opin. Food Sci. 2018;24:26–35. doi: 10.1016/j.cofs.2018.10.013. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

