Inoculation and Screening Methods for Major Sorghum Diseases Caused by Fungal Pathogens: Claviceps africana, Colletotrichum sublineola, Sporisorium reilianum, Peronosclerospora sorghi and Macrophomina phaseolina
- PMID: 37176964
- PMCID: PMC10180756
- DOI: 10.3390/plants12091906
Inoculation and Screening Methods for Major Sorghum Diseases Caused by Fungal Pathogens: Claviceps africana, Colletotrichum sublineola, Sporisorium reilianum, Peronosclerospora sorghi and Macrophomina phaseolina
Abstract
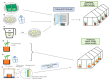
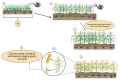
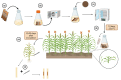
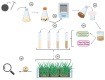
Sorghum is the fifth most important crop globally. Researching interactions between sorghum and fungal pathogens is essential to further elucidate plant defense mechanisms to biotic stress, which allows breeders to employ genetic resistance to disease. A variety of creative and useful inoculation and screening methods have been developed by sorghum pathologists to study major fungal diseases. As inoculation and screening methods can be keys for successfully conducting experiments, it is necessary to summarize the techniques developed by this research community. Among many fungal pathogens of sorghum, here we summarize inoculation and screening methods for five important fungal pathogens of sorghum: Claviceps africana, Colletotrichum sublineola, Sporisorium reilianum, Peronosclerospora sorghi and Macrophomina phaseolina. The methods described within will be useful for researchers who are interested in exploring sorghum-fungal pathogen interactions. Finally, we discuss the latest biotechnologies and methods for studying plant-fungal pathogen interactions and their applicability to sorghum pathology.
Keywords: Claviceps africana; Colletotrichum sublineola; Macrophomina phaseolina; Peronosclerospora sorghi; Sporisorium reilianum; disease evaluation; disease screening; fungal pathogens; pathogen inoculation; sorghum.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have influenced the work reported in this paper.
Figures
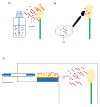
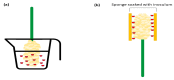

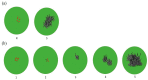
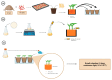

References
-
- Pažoutová S., Frederickson D.E. Genetic diversity of Claviceps africana on sorghum and Hyparrhenia. Plant Pathol. 2005;54:749–763. doi: 10.1111/j.1365-3059.2005.01255.x. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

