A Biodegradable, Polymer-Supported Oxygen Atom Transfer Reagent
- PMID: 37177199
- PMCID: PMC10181130
- DOI: 10.3390/polym15092052
A Biodegradable, Polymer-Supported Oxygen Atom Transfer Reagent
Abstract
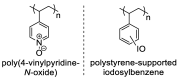
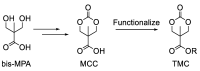
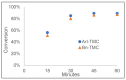
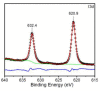
Biodegradable polymers are desirable to mitigate the environmental impact of plastic waste in the environment. Over the past several decades, the development of organocatalytic ring-opening polymerization (OROP) has made the synthesis of many new types of biodegradable polymers possible. In this research article, the first example of an oxygen atom transfer reagent pendant on a biodegradable polymer backbone is reported. The monomers for the polycarbonate backbone are sourced from the biodegradable 2,2-bis(hydroxymethyl) propionic acid molecule, and an iodoaryl group is installed pendant to the cyclic monomer for post-polymerization modification into an iodosylaryl oxygen atom transfer reagent. The key I-O bond is characterized by XPS spectroscopy, and a test reaction to triphenylphosphine demonstrates the ability of the polymer to engage in an oxygen atom transfer reaction with a substrate.
Keywords: biodegradable polymers; organocatalytic ring-opening polymerization; oxygen atom transfer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Gigmes D., Van Steenberge P.H.M., Siri D., D’hooge D.R., Guillaneuf Y., Lefay C. Simulation of the Degradation of Cyclic Ketene Acetal and Vinyl-Based Copolymers Synthesized via a Radical Process: Influence of the Reactivity Ratios on the Degradability Properties. Macromol. Rapid Commun. 2018;39:1800193. doi: 10.1002/marc.201800193. - DOI - PubMed
-
- Smit K.D., Marien Y.W., Geem K.M.V., Steenberge P.H.M.V., D’hooge D.R. Connecting Polymer Synthesis and Chemical Recycling on a Chain-by-Chain Basis: A Unified Matrix-Based Kinetic Monte Carlo Strategy. React. Chem. Eng. 2020;5:1909–1928. doi: 10.1039/D0RE00266F. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

