Recent Advancements of Polymeric Membranes in Anion Exchange Membrane Water Electrolyzer (AEMWE): A Critical Review
- PMID: 37177289
- PMCID: PMC10181302
- DOI: 10.3390/polym15092144
Recent Advancements of Polymeric Membranes in Anion Exchange Membrane Water Electrolyzer (AEMWE): A Critical Review
Abstract
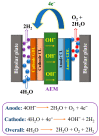
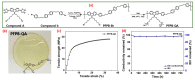
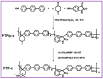
Water electrolysis coupled with renewable energy is one of the principal methods for producing green hydrogen (or renewable hydrogen). Among the different electrolysis technologies, the evolving anion exchange membrane water electrolysis (AEMWE) shows the utmost promise for the manufacture of green hydrogen in an inexpensive way. In the present review, we highlight the most current and noteworthy achievements of AEMWE, which include the advancements in increasing the polymer anionic conductivity, understanding the mechanism of degradation of AEM, and the design of the electrocatalyst. The important issues affecting the AEMWE behaviour are highlighted, and future constraints and openings are also discussed. Furthermore, this review provides strategies for producing dynamic and robust AEMWE electrocatalysts.
Keywords: AEMWE; anion exchange membrane; anionic conductivity; electrolyzer; oxygen and hydrogen evolution reaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Lee S.A., Choi S., Kim C., Yang J.W., Kim S.Y., Jang H.W. Si-based water oxidation photoanodes conjugated with earth-abundant transition metal-based catalysts. ACS Mater. Lett. 2020;2:107–126. doi: 10.1021/acsmaterialslett.9b00422. - DOI
-
- Liu J., Kang Z., Li D., Pak M., Alia S.M., Fujimoto C., Bender G., Kim Y.S., Weber A.Z. Elucidating the role of hydroxide electrolyte on anion-exchange-membrane water electrolyzer performance. J. Electrochem. Soc. 2021;168:54522. doi: 10.1149/1945-7111/ac0019. - DOI
-
- Vinodh R., Deviprasath C., Gopi C.V.V.M., Kummara V.G.R., Atchudan R., Ahamad T., Kim H.J., Yi M. Novel 13X Zeolite/PANI electrocatalyst for hydrogen and oxygen evolution reaction. Int. J. Hydrogen Energy. 2020;45:28337–28349. doi: 10.1016/j.ijhydene.2020.07.194. - DOI
-
- Gohil J.M., Dutta K. Structures and properties of polymers in ion exchange membranes for hydrogen generation by water electrolysis. Polym. Adv. Technol. 2021;32:4598–4615. doi: 10.1002/pat.5482. - DOI
-
- Nikolaidis P., Poullikkas A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017;67:597–611. doi: 10.1016/j.rser.2016.09.044. - DOI
Publication types
LinkOut - more resources
Full Text Sources

