UV-Accelerated Synthesis of Gold Nanoparticle-Pluronic Nanocomposites for X-ray Computed Tomography Contrast Enhancement
- PMID: 37177309
- PMCID: PMC10181159
- DOI: 10.3390/polym15092163
UV-Accelerated Synthesis of Gold Nanoparticle-Pluronic Nanocomposites for X-ray Computed Tomography Contrast Enhancement
Abstract
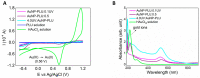
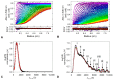
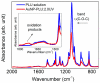
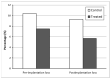
Eco-friendly chemical methods using FDA-approved Pluronic F127 (PLU) block copolymer have garnered much attention for simultaneously forming and stabilizing Au nanoparticles (AuNPs). Given the remarkable properties of AuNPs for usage in various fields, especially in biomedicine, we performed a systematic study to synthesize AuNP-PLU nanocomposites under optimized conditions using UV irradiation for accelerating the reaction. The use of UV irradiation at 254 nm resulted in several advantages over the control method conducted under ambient light (control). The AuNP-PLU-UV nanocomposite was produced six times faster, lasting 10 min, and exhibited lower size dispersion than the control. A set of experimental techniques was applied to determine the structure and morphology of the produced nanocomposites as affected by the UV irradiation. The MTT assay was conducted to estimate IC50 values of AuNP-PLU-UV in NIH 3T3 mouse embryonic fibroblasts, and the results suggest that the sample is more compatible with cells than control samples. Afterward, in vivo maternal and fetal toxicity assays were performed in rats to evaluate the effect of AuNP-PLU-UV formulation during pregnancy. Under the tested conditions, the treatment was found to be safe for the mother and fetus. As a proof of concept or application, the synthesized Au:PLU were tested as contrast agents with an X-ray computed tomography scan (X-ray CT).
Keywords: Pluronic F127; UV accelerated synthesis; X-ray computed tomography; cytotoxicity; gold nanoparticle; maternal and fetal toxicity assays; nanocomposites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


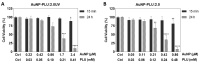


References
-
- Wei X.Z., Mulvaney P. Chapter 3—Optical Properties of Strongly Coupled Plasmonic Nanoparticle Clusters. In: Maradudin A., Sambles J.R., William L.B., editors. Modern Plasmonics. Volume 4. Elsevier; Amsterdam, The Netherlands: 2014. pp. 75–108.
-
- Csaki A., Thiele M., Jacqueline Jatschka J., Dathe A., Zopf D., Stranik O., Fritzsche W. Plasmonic nanoparticle synthesis and bioconjugation for bioanalytical sensing. Eng. Life Sci. 2015;15:266–275. doi: 10.1002/elsc.201400075. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

