Nafion: New and Old Insights into Structure and Function
- PMID: 37177360
- PMCID: PMC10181149
- DOI: 10.3390/polym15092214
Nafion: New and Old Insights into Structure and Function
Abstract
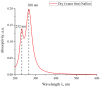
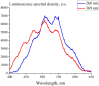
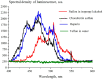
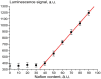
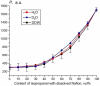
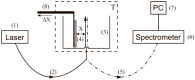
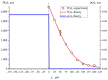
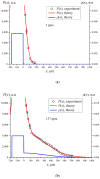
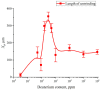
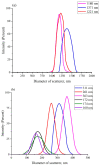
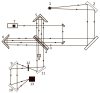
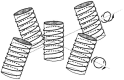
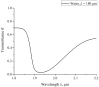
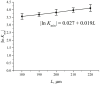
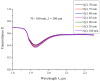
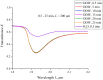
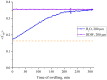
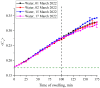
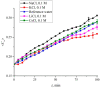
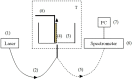
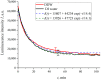
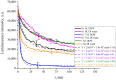
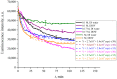
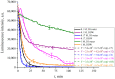
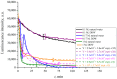
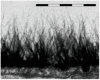
The work reports a number of results on the dynamics of swelling and inferred nanostructure of the ion-exchange polymer membrane Nafion in different aqueous solutions. The techniques used were photoluminescent and Fourier transform IR (FTIR) spectroscopy. The centers of photoluminescence were identified as the sulfonic groups localized at the ends of the perfluorovinyl ether (Teflon) groups that form the backbone of Nafion. Changes in deuterium content of water induced unexpected results revealed in the process of polymer swelling. In these experiments, deionized (DI) water (deuterium content 157 ppm) and deuterium depleted water (DDW) with deuterium content 3 PPM, were investigated. The strong hydration of sulfonic groups involves a competition between ortho- and para-magnetic forms of a water molecule. Deuterium, as it seems, adsorbs competitively on the sulfonic groups and thus can change the geometry of the sulfate bonds. With photoluminescent spectroscopy experiments, this is reflected in the unwinding of the polymer fibers into the bulk of the adjoining water on swelling. The unwound fibers do not tear off from the polymer substrate. They form a vastly extended "brush" type structure normal to the membrane surface. This may have implications for specificity of ion transport in biology, where the ubiquitous glycocalyx of cells and tissues invariably involves highly sulfated polymers such asheparan and chondroitin sulfate.
Keywords: Fourier transform IR spectroscopy; Nafion; deuterium-depleted water; endothelial surface layer; exclusion zone; fuel cells; photoluminescence spectroscopy; specific electrolyte (Hofmeister) effects; swelling of polymer membrane; unwinding of polymer fibers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Safronova E.Y., Voropaeva D.Y., Novikova S.A., Yaroslavtsev A.B. On the Influence of Solvent and Ultrasonic Treatment on Properties of Cast Nafion (R) Membranes. Membr. Technol. 2022;4:39–47. doi: 10.1134/S2517751622010073. - DOI
-
- Yu X., Chen X.D., Yu X.L., Chen X.P., Ding X., Zhao X., Tang K. Ultrahighly Sensitive QCM Humidity Sensor Based on Nafion/MoS2 Hybrid Thin Film. IEEE Trans. Electron Devices. 2022;69:1321–1326. doi: 10.1109/TED.2021.3137088. - DOI
-
- Mehmandoust M., Mehmandoust A., Erk N. Construction of a simple and selective electrochemical sensor based on Nafion/TiO2 for the voltammetric determination of olopatadine. J. Electrochem. Sci. Eng. 2022;12:91–103. doi: 10.5599/jese.1117. - DOI
Publication types
LinkOut - more resources
Full Text Sources

