An extensive survey of phytoviral RNA 3' uridylation identifies extreme variations and virus-specific patterns
- PMID: 37177985
- PMCID: PMC10469402
- DOI: 10.1093/plphys/kiad278
An extensive survey of phytoviral RNA 3' uridylation identifies extreme variations and virus-specific patterns
Abstract
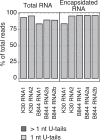
Viral RNAs can be uridylated in eukaryotic hosts. However, our knowledge of uridylation patterns and roles remains rudimentary for phytoviruses. Here, we report global 3' terminal RNA uridylation profiles for representatives of the main families of positive single-stranded RNA phytoviruses. We detected uridylation in all 47 viral RNAs investigated here, revealing its prevalence. Yet, uridylation levels of viral RNAs varied from 0.2% to 90%. Unexpectedly, most poly(A) tails of grapevine fanleaf virus (GFLV) RNAs, including encapsidated tails, were strictly monouridylated, which corresponds to an unidentified type of viral genomic RNA extremity. This monouridylation appears beneficial for GFLV because it became dominant when plants were infected with nonuridylated GFLV transcripts. We found that GFLV RNA monouridylation is independent of the known terminal uridylyltransferases (TUTases) HEN1 SUPPRESSOR 1 (HESO1) and UTP:RNA URIDYLYLTRANSFERASE 1 (URT1) in Arabidopsis (Arabidopsis thaliana). By contrast, both TUTases can uridylate other viral RNAs like turnip crinkle virus (TCV) and turnip mosaic virus (TuMV) RNAs. Interestingly, TCV and TuMV degradation intermediates were differentially uridylated by HESO1 and URT1. Although the lack of both TUTases did not prevent viral infection, we detected degradation intermediates of TCV RNA at higher levels in an Arabidopsis heso1 urt1 mutant, suggesting that uridylation participates in clearing viral RNA. Collectively, our work unveils an extreme diversity of uridylation patterns across phytoviruses and constitutes a valuable resource to further decipher pro- and antiviral roles of uridylation.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. The authors declare that there is no conflict of interest.
Figures








References
-
- Cock PJA, Antao T, Chang JT, Chapman BA, Cox CJ, Dalke A, Friedberg I, Hamelryck T, Kauff F, Wilczynski B, et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009:25(11):1422–1423. 10.1093/bioinformatics/btp163 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

