Ceramide analog C2-cer induces a loss in insulin sensitivity in muscle cells through the salvage/recycling pathway
- PMID: 37178918
- PMCID: PMC10276168
- DOI: 10.1016/j.jbc.2023.104815
Ceramide analog C2-cer induces a loss in insulin sensitivity in muscle cells through the salvage/recycling pathway
Abstract
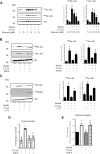
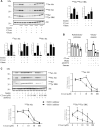
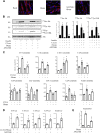
Ceramides have been shown to play a major role in the onset of skeletal muscle insulin resistance and therefore in the prevalence of type 2 diabetes. However, many of the studies involved in the discovery of deleterious ceramide actions used a nonphysiological, cell-permeable, short-chain ceramide analog, the C2-ceramide (C2-cer). In the present study, we determined how C2-cer promotes insulin resistance in muscle cells. We demonstrate that C2-cer enters the salvage/recycling pathway and becomes deacylated, yielding sphingosine, re-acylation of which depends on the availability of long chain fatty acids provided by the lipogenesis pathway in muscle cells. Importantly, we show these salvaged ceramides are actually responsible for the inhibition of insulin signaling induced by C2-cer. Interestingly, we also show that the exogenous and endogenous monounsaturated fatty acid oleate prevents C2-cer to be recycled into endogenous ceramide species in a diacylglycerol O-acyltransferase 1-dependent mechanism, which forces free fatty acid metabolism towards triacylglyceride production. Altogether, the study highlights for the first time that C2-cer induces a loss in insulin sensitivity through the salvage/recycling pathway in muscle cells. This study also validates C2-cer as a convenient tool to decipher mechanisms by which long-chain ceramides mediate insulin resistance in muscle cells and suggests that in addition to the de novo ceramide synthesis, recycling of ceramide could contribute to muscle insulin resistance observed in obesity and type 2 diabetes.
Keywords: Akt PKB; cell signaling; diacylglycerol; lipid signaling; lipogenesis; lipotoxicity; metabolism; oleate; signal transduction.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures
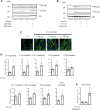
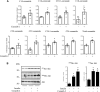
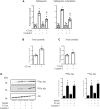
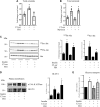
References
-
- Katz L.D., Glickman M.G., Rapoport S., Ferrannini E., DeFronzo R.A. Splanchnic and peripheral disposal of oral glucose in man. Diabetes. 1983;32:675–679. - PubMed
-
- Charles M.A., Eschwege E., Thibult N., Claude J.R., Warnet J.M., Rosselin G.E., et al. The role of non-esterified fatty acids in the deterioration of glucose tolerance in caucasian subjects: results of the paris prospective study. Diabetologia. 1997;40:1101–1106. - PubMed
-
- Zheng W., Kollmeyer J., Symolon H., Momin A., Munter E., Wang E., et al. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochim. Biophys. Acta. 2006;1758:1864–1884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

