Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications
- PMID: 37179402
- PMCID: PMC10183032
- DOI: 10.1038/s41392-023-01406-7
Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications
Abstract
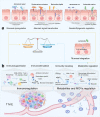
In the past period, due to the rapid development of next-generation sequencing technology, accumulating evidence has clarified the complex role of the human microbiota in the development of cancer and the therapeutic response. More importantly, available evidence seems to indicate that modulating the composition of the gut microbiota to improve the efficacy of anti-cancer drugs may be feasible. However, intricate complexities exist, and a deep and comprehensive understanding of how the human microbiota interacts with cancer is critical to realize its full potential in cancer treatment. The purpose of this review is to summarize the initial clues on molecular mechanisms regarding the mutual effects between the gut microbiota and cancer development, and to highlight the relationship between gut microbes and the efficacy of immunotherapy, chemotherapy, radiation therapy and cancer surgery, which may provide insights into the formulation of individualized therapeutic strategies for cancer management. In addition, the current and emerging microbial interventions for cancer therapy as well as their clinical applications are summarized. Although many challenges remain for now, the great importance and full potential of the gut microbiota cannot be overstated for the development of individualized anti-cancer strategies, and it is necessary to explore a holistic approach that incorporates microbial modulation therapy in cancer.
© 2023. The Author(s).
Conflict of interest statement
The authors no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

