Engineered dual affinity protein fragments to bind collagen and capture growth factors
- PMID: 37179535
- PMCID: PMC10173277
- DOI: 10.1016/j.mtbio.2023.100641
Engineered dual affinity protein fragments to bind collagen and capture growth factors
Abstract
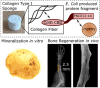
Collagen type I lacks affinity for growth factors (GFs) and yet it is clinically used to deliver bone morphogenic protein 2 (BMP-2), a potent osteogenic growth factor. To mitigate this lack of affinity, supra-physiological concentrations of BMP-2 are loaded in collagen sponges leading to uncontrolled BMP-2 leakage out of the material. This has led to important adverse side effects such as carcinogenesis. Here, we design recombinant dual affinity protein fragments, produced in E. Coli, which contain two regions, one that spontaneously binds to collagen and a second one that binds BMP-2. By adding the fragment to collagen sponges, BMP-2 is sequestered enabling solid phase presentation of BMP-2. We demonstrate osteogenesis in vivo with ultra-low doses of BMP-2. Our protein technology enhances the biological activity of collagen without using complex chemistries or changing the manufacturing of the base material and so opens a pathway to clinical translation.
Keywords: Bacteria; Biomaterials; Bone regeneration; Collagen; Fibronectin; Human mesenchymal stem cells; Recombinant protein fragment.
© 2023 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Manuel Salmeron-Sanchez reports financial support was provided by 10.13039/501100000853University of Glasgow.
Figures

References
LinkOut - more resources
Full Text Sources

