Designed NiMoC@C and NiFeMo2C@C core-shell nanoparticles for oxygen evolution in alkaline media
- PMID: 37179773
- PMCID: PMC10169681
- DOI: 10.3389/fchem.2023.1162675
Designed NiMoC@C and NiFeMo2C@C core-shell nanoparticles for oxygen evolution in alkaline media
Abstract
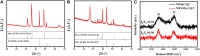
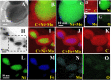
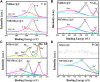
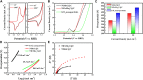
Electrochemical water splitting is one of the most promising and clean ways to produce hydrogen as a fuel. Herein, we present a facile and versatile strategy for synthesizing non-precious transition binary and ternary metal-based catalysts encapsulated in a graphitic carbon shell. NiMoC@C and NiFeMo2C@C were prepared via a simple sol-gel based method for application in the Oxygen Evolution Reaction (OER). The conductive carbon layer surrounding the metals was introduced to improve electron transport throughout the catalyst structure. This multifunctional structure showed synergistic effects, possess a larger number of active sites and enhanced electrochemical durability. Structural analysis indicated that the metallic phases were encapsulated in the graphitic shell. Experimental results demonstrated that the optimal core-shell material NiFeMo2C@C exhibited the best catalytic performance for the OER in 0.5 M KOH, reaching a current density of 10 mA cm-2 at low overpotential of 292 mV for the OER, superior to the benchmark IrO2 nanoparticles. The good performances and stability of these OER electrocatalysts, alongside an easily scalable procedure makes these systems ideal for industrial purposes.
Keywords: OER; binary transition metal; core-shell structure; graphitic carbon; metal-metal carbide hybrid; nanoparticles; ternary transition metal; urea glass route.
Copyright © 2023 Li and Giordano.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor [DR] declared a past co-authorship with the authors [CG].
Figures



References
-
- Ahsan M. A., Puente Santiago A. R., Hong Y., Zhang N., Cano M., Rodriguez-Castellon E., et al. (2020). Tuning of trifunctional NiCu bimetallic nanoparticles confined in a porous carbon Network with surface composition and local structural distortions for the electrocatalytic oxygen reduction, oxygen and hydrogen evolution reactions. J. Am. Chem. Soc. 142 (34), 14688–14701. 10.1021/jacs.0c06960 - DOI - PubMed
-
- An L., Yang F., Fu C., Cai X., Shen S., Xia G., et al. (2022). A functionally stable RuMn electrocatalyst for oxygen evolution reaction in acid. Adv. Funct. Mater. 32 (27), 2200131. 10.1002/adfm.202200131 - DOI
-
- Badrnezhad R., Nasri F., Pourfarzad H., Jafari S. K. (2021). Effect of iron on Ni–Mo–Fe composite as a low-cost bifunctional electrocatalyst for overall water splitting. Int. J. Hydrogen Energy 46 (5), 3821–3832. 10.1016/j.ijhydene.2020.10.174 - DOI
-
- Bancroft H. W. N. Á. D. L. Á. G. M., Legrand D., Bancroft G. M. (2000). Interpretation of Ni2p XPS spectra of Ni conductors and Ni insulators. Phys. Chem. Miner. 27, 357–366. 10.1007/s002690050265 - DOI
LinkOut - more resources
Full Text Sources

