A systematic review and meta-analysis of CD22 CAR T-cells alone or in combination with CD19 CAR T-cells
- PMID: 37180149
- PMCID: PMC10174241
- DOI: 10.3389/fimmu.2023.1178403
A systematic review and meta-analysis of CD22 CAR T-cells alone or in combination with CD19 CAR T-cells
Abstract
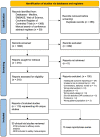
Chimeric antigen receptor (CAR) T-cells are an emerging therapy for the treatment of relapsed/refractory B-cell malignancies. While CD19 CAR-T cells have been FDA-approved, CAR T-cells targeting CD22, as well as dual-targeting CD19/CD22 CAR T-cells, are currently being evaluated in clinical trials. This systematic review and meta-analysis aimed to evaluate the efficacy and safety of CD22-targeting CAR T-cell therapies. We searched MEDLINE, EMBASE, Web of Science, and the Cochrane Central Register of Controlled Trials from inception to March 3rd 2022 for full-length articles and conference abstracts of clinical trials employing CD22-targeting CAR T-cells in acute lymphocytic leukemia (ALL) and non-Hodgkin's lymphoma (NHL). The primary outcome was best complete response (bCR). A DerSimonian and Laird random-effects model with arcsine transformation was used to pool outcome proportions. From 1068 references screened, 100 were included, representing 30 early phase studies with 637 patients, investigating CD22 or CD19/CD22 CAR T-cells. CD22 CAR T-cells had a bCR of 68% [95% CI, 53-81%] in ALL (n= 116), and 64% [95% CI, 46-81%] in NHL (n= 28) with 74% and 96% of patients having received anti-CD19 CAR T-cells previously in ALL and NHL studies respectively. CD19/CD22 CAR T-cells had a bCR rate of 90% [95% CI, 84-95%] in ALL (n= 297) and 47% [95% CI, 34-61%] in NHL (n= 137). The estimated incidence of total and severe (grade ≥3) CRS were 87% [95% CI, 80-92%] and 6% [95% CI, 3-9%] respectively. ICANS and severe ICANS had an estimated incidence of 16% [95% CI, 9-25%] and 3% [95% CI, 1-5%] respectively. Early phase trials of CD22 and CD19/CD22 CAR T-cells show high remission rates in ALL and NHL. Severe CRS or ICANS were (1)rare and dual-targeting did not increase toxicity. Variability in CAR construct, dose, and patient factors amongst studies limits comparisons, with long-term outcomes yet to be reported.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier CRD42020193027.
Keywords: B-cell malignancies; CAR T-cell; CD22; efficacy; safety; systematic review & meta-analysis.
Copyright © 2023 Fergusson, Adeel, Kekre, Atkins and Hay.
Conflict of interest statement
KH serves on advisory board for Kite/Gilead, Cellgene/BMS, Novartis, Janssen and receives payments for Jazz Pharmaceutical educational programs. KH has also received research funding from Janssen. NK serves on advisory board for Kite/Gilead, Cellgene/BMS, and Novartis. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures



References
-
- Gokbuget N, Dombret H, Ribera JM, Fielding AK, Advani A, Bassan R, et al. . International reference analysis of outcomes in adults with b-precursor ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica (2016) 101(12):1524–33. doi: 10.3324/haematol.2016.144311 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

