Future of genetic therapies for rare genetic diseases: what to expect for the next 15 years?
- PMID: 37180410
- PMCID: PMC10032453
- DOI: 10.1177/26330040221100840
Future of genetic therapies for rare genetic diseases: what to expect for the next 15 years?
Abstract
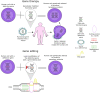
Introduction: Rare genetic diseases affect millions of people worldwide. Most of them are caused by defective genes that impair quality of life and can lead to premature death. As genetic therapies aim to fix or replace defective genes, they are considered the most promising treatment for rare genetic diseases. Yet, as these therapies are still under development, it is still unclear whether they will be successful in treating these diseases. This study aims to address this gap by assessing researchers' opinions on the future of genetic therapies for the treatment of rare genetic diseases.
Methods: We conducted a global cross-sectional web-based survey of researchers who recently authored peer-reviewed articles related to rare genetic diseases.
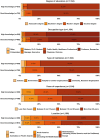
Results: We assessed the opinions of 1430 researchers with high and good knowledge about genetic therapies for the treatment of rare genetic diseases. Overall, the respondents believed that genetic therapies would be the standard of care for rare genetic diseases before 2036, leading to cures after this period. CRISPR-Cas9 was considered the most likely approach to fixing or replacing defective genes in the next 15 years. The respondents with good knowledge believed that genetic therapies would only have long-lasting effects after 2036, while those with high knowledge were divided on this issue. The respondents with good knowledge on the subject believed that non-viral vectors are more likely to be successful in fixing or replacing defective genes in the next 15 years, while most of the respondents with high knowledge believed viral vectors would be more successful.
Conclusion: Overall, the researchers who participated in this study expect that in the future genetic therapies will greatly benefit the treatment of patients with rare genetic diseases.
Keywords: expert opinion; future; genetic therapies; rare genetic diseases; survey.
Plain language summary
A global survey of researchers on the future of genetic therapies for rare genetic diseases Rare genetic diseases are caused by defective genes that result from one or more mutations in the genome. Today, the therapeutic options for these diseases are limited, and there are approved treatments for about 5% of them. In the future, genetic therapies (a group of techniques developed to correct defective genes) are expected to revolutionize the treatment of rare genetic diseases. Although promising, most of these therapies are currently under development and have a long way to go before their efficacy and safety can be proved. The uncertainty surrounding this topic therefore means the success of genetic therapies in treating or curing rare genetic diseases is not yet assured. To address this knowledge gap, we surveyed 1430 researchers working in rare genetic diseases about the future of genetic therapies for the treatment of these diseases over the next 15 years. Most of them expected gene therapies to be the standard of care for rare genetic diseases before 2036 and to be able to cure them after this date. CRISPR-Cas9 was felt to be the gene editing approach that was most likely to succeed in fixing or replacing defective genes in the next 15 years. The respondents with high knowledge about gene therapies for the treatment of rare diseases believed gene therapies would have long-lasting effects before 2036, while those with good knowledge expected this to be the case only after 2036. The former believed in viral vectors and the latter in non-viral vectors to fix or replace defective genes in the next 15 years.
© The Author(s), 2022.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures



References
-
- Delhove J, Osenk I, Prichard I, et al. Public acceptability of gene therapy and gene editing for human use: a systematic review. Hum Gene Ther 2020; 31: 20–46. - PubMed
-
- Kole A, Hedley V.Recommendations from the rare 2030 foresight study: the future of rare diseases starts today, EURORDIS, Europe 2021, https://download2.eurordis.org/rare2030/Rare2030_recommendations.pdf
-
- Roessler HI, Knoers NVAM, van Haelst MM, et al. Drug repurposing for rare diseases. Trends Pharmacol Sci 2021; 42: 255–267. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
