Variant rs8400 enhances ALKBH5 expression through disrupting miR-186 binding and promotes neuroblastoma progression
- PMID: 37180836
- PMCID: PMC10167609
- DOI: 10.21147/j.issn.1000-9604.2023.02.05
Variant rs8400 enhances ALKBH5 expression through disrupting miR-186 binding and promotes neuroblastoma progression
Abstract
Objective: AlkB homolog 5 (ALKBH5) has been proven to be closely related to tumors. However, the role and molecular mechanism of ALKBH5 in neuroblastomas have rarely been reported.
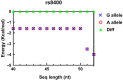
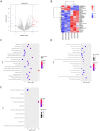
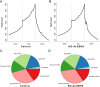
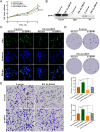
Methods: The potential functional single-nucleotide polymorphisms (SNPs) in ALKBH5 were identified by National Center for Biotechnology Information (NCBI) dbSNP screening and SNPinfo software. TaqMan probes were used for genotyping. A multiple logistic regression model was used to evaluate the effects of different SNP loci on the risk of neuroblastoma. The expression of ALKBH5 in neuroblastoma was evaluated by Western blotting and immunohistochemistry (IHC). Cell counting kit-8 (CCK-8), plate colony formation and 5-ethynyl-2'-deoxyuridine (EdU) incorporation assays were used to evaluate cell proliferation. Wound healing and Transwell assays were used to compare cell migration and invasion. Thermodynamic modelling was performed to predict the ability of miRNAs to bind to ALKBH5 with the rs8400 G/A polymorphism. RNA sequencing, N6-methyladenosine (m6A) sequencing, m6A methylated RNA immunoprecipitation (MeRIP) and a luciferase assay were used to identify the targeting effect of ALKBH5 on SPP1.
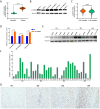
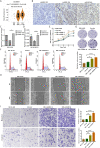
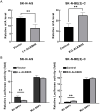
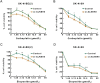
Results: ALKBH5 was highly expressed in neuroblastoma. Knocking down ALKBH5 inhibited the proliferation, migration and invasion of cancer cells. miR-186-3p negatively regulates the expression of ALKBH5, and this ability is affected by the rs8400 polymorphism. When the G nucleotide was mutated to A, the ability of miR-186-3p to bind to the 3'-UTR of ALKBH5 decreased, resulting in upregulation of ALKBH5. SPP1 is the downstream target gene of the ALKBH5 oncogene. Knocking down SPP1 partially restored the inhibitory effect of ALKBH5 downregulation on neuroblastoma. Downregulation of ALKBH5 can improve the therapeutic efficacy of carboplatin and etoposide in neuroblastoma.
Conclusions: We first found that the rs8400 G>A polymorphism in the m6A demethylase-encoding gene ALKBH5 increases neuroblastoma susceptibility and determines the related mechanisms. The aberrant regulation of ALKBH5 by miR-186-3p caused by this genetic variation in ALKBH5 promotes the occurrence and development of neuroblastoma through the ALKBH5-SPP1 axis.
Keywords: ALKBH5; Neuroblastoma; polymorphism; progression; risk.
Copyright ©2023 Chinese Journal of Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures






References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
