Bile acid interactions with neurotransmitter transporters
- PMID: 37180953
- PMCID: PMC10169653
- DOI: 10.3389/fncel.2023.1161930
Bile acid interactions with neurotransmitter transporters
Abstract
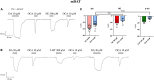
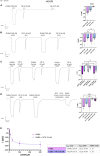
Synthesized in the liver from cholesterol, the bile acids (BAs) primary role is emulsifying fats to facilitate their absorption. BAs can cross the blood-brain barrier (BBB) and be synthesized in the brain. Recent evidence suggests a role for BAs in the gut-brain signaling by modulating the activity of various neuronal receptors and transporters, including the dopamine transporter (DAT). In this study, we investigated the effects of BAs and their relationship with substrates in three transporters of the solute carrier 6 family. The exposure to obeticholic acid (OCA), a semi-synthetic BA, elicits an inward current (IBA) in the DAT, the GABA transporter 1 (GAT1), and the glycine transporter 1 (GlyT1b); this current is proportional to the current generated by the substrate, respective to the transporter. Interestingly, a second consecutive OCA application to the transporter fails to elicit a response. The full displacement of BAs from the transporter occurs only after exposure to a saturating concentration of a substrate. In DAT, perfusion of secondary substrates norepinephrine (NE) and serotonin (5-HT) results in a second OCA current, decreased in amplitude and proportional to their affinity. Moreover, co-application of 5-HT or NE with OCA in DAT, and GABA with OCA in GAT1, did not alter the apparent affinity or the Imax, similar to what was previously reported in DAT in the presence of DA and OCA. The findings support the previous molecular model that suggested the ability of BAs to lock the transporter in an occluded conformation. The physiological significance is that it could possibly avoid the accumulation of small depolarizations in the cells expressing the neurotransmitter transporter. This achieves better transport efficiency in the presence of a saturating concentration of the neurotransmitter and enhances the action of the neurotransmitter on their receptors when they are present at reduced concentrations due to decreased availability of transporters.
Keywords: GABA transporter 1; SLC6; bile acids; dopamine transporter; glycine transporter; obeticholic acid (OCA); two-electrode voltage clamp.
Copyright © 2023 Romanazzi, Zanella, Bhatt, Di Iacovo, Galli and Bossi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

