Stereoselective synthesis of (E)-α,β-unsaturated esters: triethylamine-catalyzed allylic rearrangement of enol phosphates
- PMID: 37181505
- PMCID: PMC10173029
- DOI: 10.1039/d3ra02430j
Stereoselective synthesis of (E)-α,β-unsaturated esters: triethylamine-catalyzed allylic rearrangement of enol phosphates
Abstract
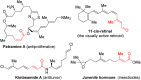
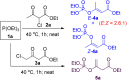
α,β-Unsaturated esters are key structural motifs widely distributed in various biologically active molecules, and their Z/E-stereoselective synthesis has always been considered highly attractive in organic synthesis. Herein, we present a >99% (E)-stereoselective one-pot synthetic approach towards β-phosphoroxylated α,β-unsaturated esters via a mild trimethylamine-catalyzed 1,3-hydrogen migration of the corresponding unconjugated intermediates derived from the solvent-free Perkow reaction between low-cost 4-chloroacetoacetates and phosphites. Versatile β,β-disubstituted (E)-α,β-unsaturated esters were thus afforded with full (E)-stereoretentivity by cleavage of the phosphoenol linkage via Negishi cross-coupling. Moreover, a stereoretentive (E)-rich mixture of a α,β-unsaturated ester derived from 2-chloroacetoacetate was obtained and both isomers were easily afforded in one operation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
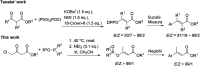


Similar articles
-
Stereocomplementary and Parallel Syntheses of Multi-Substituted (E)-, (Z)-Stereodefined α,β-Unsaturated Esters: Application to Drug Syntheses.Chem Rec. 2020 Dec;20(12):1410-1429. doi: 10.1002/tcr.202000076. Epub 2020 Sep 15. Chem Rec. 2020. PMID: 32931154 Review.
-
(E)- and (Z)-stereodefined enol phosphonates derived from β-ketoesters: stereocomplementary synthesis of fully-substituted α,β-unsaturated esters.Org Biomol Chem. 2015 Aug 14;13(30):8205-10. doi: 10.1039/c5ob01097g. Org Biomol Chem. 2015. PMID: 26133291
-
Highly stereoselective synthesis of (E)- and (Z)-alpha-fluoro-alpha,beta-unsaturated esters and (E)- and (Z)-alpha-fluoro-alpha,beta-unsaturated amides from 1-bromo-1-fluoroalkenes via palladium-catalyzed carbonylation reactions.J Org Chem. 2005 May 27;70(11):4346-53. doi: 10.1021/jo050314a. J Org Chem. 2005. PMID: 15903310
-
General, robust, and stereocomplementary preparation of alpha,beta-disubstituted alpha,beta-unsaturated esters.Org Lett. 2009 Oct 1;11(19):4258-61. doi: 10.1021/ol9013359. Org Lett. 2009. PMID: 19715286
-
[Development of highly stereoselective reactions utilizing heteroatoms--new approach to the stereoselective Horner-Wadsworth-Emmons reaction].Yakugaku Zasshi. 2000 May;120(5):432-44. doi: 10.1248/yakushi1947.120.5_432. Yakugaku Zasshi. 2000. PMID: 10825807 Review. Japanese.
Cited by
-
Organophosphates as Versatile Substrates in Organic Synthesis.Molecules. 2024 Apr 2;29(7):1593. doi: 10.3390/molecules29071593. Molecules. 2024. PMID: 38611872 Free PMC article. Review.
References
-
- Zhuo C. X. Furstner A. J. Am. Chem. Soc. 2018;140:10514. - PubMed
- Romo D. Choi N. S. Li S. Buchler I. Shi Z. Liu J. O. J. Am. Chem. Soc. 2004;126:10582. - PubMed
- Marco S. D. Cammas A. Pelletier J. Gallouzi I. E. Nat. Commun. 2012;3:8963. - PMC - PubMed
- Naineni S. K. Liang J. Nagar B. Pelletier J. Cell Chem. Biol. 2021;28:825. - PMC - PubMed
-
- Guo P. Zhang Y. Zhang L. Xia Q. J. Biol. Chem. 2021;297:101234. - PMC - PubMed
- Ando Y. Matsumoto K. Misaki K. Mano G. Shinada T. Goto S. G. Gen. Comp. Endocrinol. 2020;289:113394. - PubMed
- Nouzova M. Rivera-Pérez C. Noriega F. G. Curr. Opin. Insect. Sci. 2018;29:49. - PMC - PubMed
- Li K. Jia Q. Li S. Insect Sci. 2019;26:600. - PubMed
-
- Evans D. A. Coleman P. J. Dias L. C. Tyler A. N. Angew. Chem., Int. Ed. Engl. 1997;36:2744.
- Evans D. A. Fitch D. M. Smith T. E. Cee V. J. Am. Chem. Soc. 2000;122:10033.
- Evans D. A. Carter P. H. Carreira E. M. Charette A. B. Prunet J. A. Lautens M. J. Am. Chem. Soc. 1999;121:7540.
- Fleming I. Barbero A. Walter D. Chem. Rev. 1997;97:2063. - PubMed
LinkOut - more resources
Full Text Sources

