Stability of carbon quantum dots: a critical review
- PMID: 37181523
- PMCID: PMC10167674
- DOI: 10.1039/d2ra07180k
Stability of carbon quantum dots: a critical review
Abstract
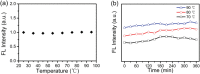
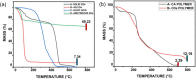
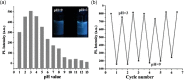
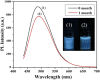
Carbon quantum dots (CQDs) are fluorescent carbon nanomaterials with unique optical and structural properties that have drawn extensive attention from researchers in the past few decades. Environmental friendliness, biocompatibility and cost effectiveness of CQDs have made them very renowned in countless applications including solar cells, white light-emitting diodes, bio-imaging, chemical sensing, drug delivery, environmental monitoring, electrocatalysis, photocatalysis and other related areas. This review is explicitly dedicated to the stability of CQDs under different ambient conditions. Stability of CQDs is very important for every possible application and no review has been put forth to date that emphasises it, to the best of our knowledge. This review's primary goal is to make the readers cognizant of the importance of stability, ways to assess it, factors that affect it and proposed ways to enhance the stability for making CQDs suitable for commercial applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
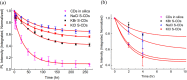
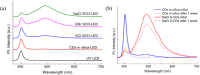
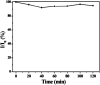

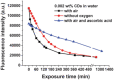

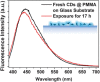

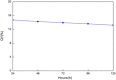
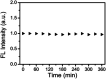

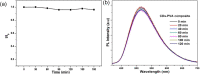









References
-
- Magesh V. Ashok K. Sundramoorthy, Dhanraj Ganapathy, Recent Advances on Synthesis and Potential Applications of Carbon Quantum Dots. Front. Mater. 2022;9:1–27. doi: 10.3389/fmats.2022.906838. - DOI
-
- Azam N. Ali M. N. Khan T. J. Carbon Quantum Dots for Biomedical Applications: Review and Analysis. Front. Mater. 2021;8:1–21. doi: 10.3389/fmats.2021.700403. - DOI
-
- Zhang W. Yu S. F. Fei L. F. Jin L. Pan S. Lin P. Large area color controllable remote carbon white-light light emitting diodes. Carbon. 2015;85:344–350. doi: 10.1016/j.carbon.2014.12.107. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

