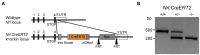
Nrl:CreERT2 mouse model to induce mosaic gene expression in rod photoreceptors
- PMID: 37181654
- PMCID: PMC10166802
- DOI: 10.3389/fnmol.2023.1161127
Nrl:CreERT2 mouse model to induce mosaic gene expression in rod photoreceptors
Abstract
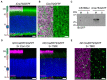
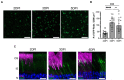
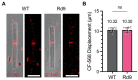
Photoreceptors are sensory neurons that capture light within their outer segment, a narrow cylindrical organelle stacked with disc-shaped membranes housing the visual pigment. Photoreceptors are the most abundant neurons in the retina and are tightly packed to maximize the capture of incoming light. As a result, it is challenging to visualize an individual cell within a crowded photoreceptor population. To address this limitation, we developed a rod-specific mouse model that expresses tamoxifen-inducible cre recombinase under the control of the Nrl promoter. We characterized this mouse using a farnyslated GFP (GFPf) reporter mouse and found mosaic rod expression throughout the retina. The number of GFPf-expressing rods stabilized within 3 days post tamoxifen injection. At that time, the GFPf reporter began to accumulate in basal disc membranes. Using this new reporter mouse, we attempted to quantify the time course of photoreceptor disc renewal in WT and Rd9 mice, a model of X-linked retinitis pigmentosa previously proposed to have a reduced disc renewal rate. We measured GFPf accumulation in individual outer segments at 3 and 6 days post-induction and found that basal accumulation of the GFPf reporter was unchanged between WT and Rd9 mice. However, rates of renewal based on the GFPf measurements were inconsistent with historical calculations from radiolabeled pulse-chase experiments. By extending GFPf reporter accumulation to 10 and 13 days we found that this reporter had an unexpected distribution pattern that preferentially labeled the basal region of the outer segment. For these reasons the GFPf reporter cannot be used for measuring rates of disc renewal. Therefore, we used an alternative method that labels newly forming discs with a fluorescent dye to measure disc renewal rates directly in the Rd9 model and found it was not significantly different from WT. Our study finds that the Rd9 mouse has normal rates of disc renewal and introduces a novel Nrl:CreERT2 mouse for gene manipulation of individual rods.
Keywords: NRL; RD9; disc renewal; inducible; outer segment; photoreceptor; retinal degeneration.
Copyright © 2023 Thorson, Wei, Johnson, Gabriel, Arshavsky and Pearring.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bates D., Machler M., Bolker B., Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01 - DOI
-
- Besharse J. C., Hollyfield J. G. (1979). Turnover of mouse photoreceptor outer segments in constant light and darkness. Invest. Ophthalmol. Vis. Sci. 18, 1019–1024. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

