Stress Granules as Causes and Consequences of Translation Suppression
- PMID: 37183403
- PMCID: PMC10443205
- DOI: 10.1089/ars.2022.0164
Stress Granules as Causes and Consequences of Translation Suppression
Abstract
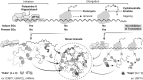
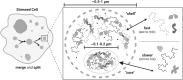
Significance: Stress granules (SGs) are biomolecular condensates that form upon global translation suppression during stress. SGs are enriched in translation factors and messenger RNAs (mRNAs), which they may sequester away from the protein synthesis machinery. While this is hypothesized to remodel the functional transcriptome during stress, it remains unclear whether SGs are a cause, or simply a consequence, of translation repression. Understanding the function of SGs is particularly important because they are implicated in numerous diseases including viral infections, cancer, and neurodegeneration. Recent Advances: We synthesize recent SG research spanning biological scales, from observing single proteins and mRNAs within one cell to measurements of the entire transcriptome or proteome of SGs in a cell population. We use the emerging understanding from these studies to suggest that SGs likely have less impact on global translation, but instead may strongly influence the translation of individual mRNAs localized to them. Critical Issues: Development of a unified model that links stress-induced RNA-protein condensation to regulation of downstream gene expression holds promise for understanding the mechanisms of cellular resilience. Future Directions: Therefore, upcoming research should clarify what influence SGs exert on translation at all scales as well as the molecular mechanisms that enable this. The resulting knowledge will be required to drive discovery in how SGs allow organisms to adapt to challenges and support health or go awry and lead to disease. Antioxid. Redox Signal. 39, 390-409.
Keywords: RNP granules; biomolecular condensates; stress granules; stress response; translation.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

