Advances in understanding the function of alpha-synuclein: implications for Parkinson's disease
- PMID: 37183455
- PMCID: PMC10473562
- DOI: 10.1093/brain/awad150
Advances in understanding the function of alpha-synuclein: implications for Parkinson's disease
Abstract
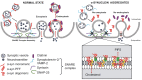
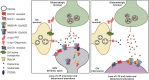
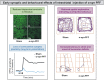
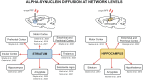
The critical role of alpha-synuclein in Parkinson's disease represents a pivotal discovery. Some progress has been made over recent years in identifying disease-modifying therapies for Parkinson's disease that target alpha-synuclein. However, these treatments have not yet shown clear efficacy in slowing the progression of this disease. Several explanations exist for this issue. The pathogenesis of Parkinson's disease is complex and not yet fully clarified and the heterogeneity of the disease, with diverse genetic susceptibility and risk factors and different clinical courses, adds further complexity. Thus, a deep understanding of alpha-synuclein physiological and pathophysiological functions is crucial. In this review, we first describe the cellular and animal models developed over recent years to study the physiological and pathological roles of this protein, including transgenic techniques, use of viral vectors and intracerebral injections of alpha-synuclein fibrils. We then provide evidence that these tools are crucial for modelling Parkinson's disease pathogenesis, causing protein misfolding and aggregation, synaptic dysfunction, brain plasticity impairment and cell-to-cell spreading of alpha-synuclein species. In particular, we focus on the possibility of dissecting the pre- and postsynaptic effects of alpha-synuclein in both physiological and pathological conditions. Finally, we show how vulnerability of specific neuronal cell types may facilitate systemic dysfunctions leading to multiple network alterations. These functional alterations underlie diverse motor and non-motor manifestations of Parkinson's disease that occur before overt neurodegeneration. However, we now understand that therapeutic targeting of alpha-synuclein in Parkinson's disease patients requires caution, since this protein exerts important physiological synaptic functions. Moreover, the interactions of alpha-synuclein with other molecules may induce synergistic detrimental effects. Thus, targeting only alpha-synuclein might not be enough. Combined therapies should be considered in the future.
Keywords: Parkinson’s disease; alpha-synuclein; dopamine; striatum; synaptic plasticity.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
P.C. received/receives research support, speaker honoraria, and support to attend national and international conferences (not related to the present study) from: Abbvie, Bial, Bayer Schering, Biogen-Dompè, Biogen-Idec, Eisai, Lilly, Lundbeck, Lusofarmaco, Merck-Serono, Novartis, Sanofi-Genzyme, Teva, UCB Pharma, Zambon. The other authors report no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

