Frontotemporal Degeneration with Transactive Response DNA-Binding Protein Type C at the Anterior Temporal Lobe
- PMID: 37183762
- PMCID: PMC10330481
- DOI: 10.1002/ana.26677
Frontotemporal Degeneration with Transactive Response DNA-Binding Protein Type C at the Anterior Temporal Lobe
Abstract
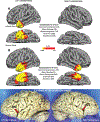
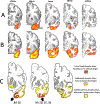
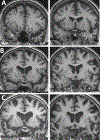
The anatomical distribution of most neurodegenerative diseases shows considerable interindividual variations. In contrast, frontotemporal lobar degeneration with transactive response DNA-binding protein type C (TDP-C) shows a consistent predilection for the anterior temporal lobe (ATL). The relatively selective atrophy of ATL in TDP-C patients has highlighted the importance of this region for complex cognitive and behavioral functions. This review includes observations on 28 TDP-C patients, 18 with semantic primary progressive aphasia and 10 with other syndromes. Longitudinal imaging allowed the delineation of progression trajectories. At post-mortem examination, the pathognomonic feature of TDP-C consisted of long, thick neurites found predominantly in superficial cortical layers. These neurites may represent dystrophic apical dendrites of layer III and V pyramidal neurons that are known to play pivotal roles in complex cortical computations. Other types of frontotemporal lobar degeneration TDP, such as TDP-A and TDP-B, are not associated with long dystrophic neurites in the cerebral cortex, and do not show similar predilection patterns for ATL. Research is beginning to identify molecular, structural, and immunological differences between pathological TDP-43 in TDP-C versus TDP-A and B. Parallel investigations based on proteomics, somatic mutations, and genome-wide association studies are detecting molecular features that could conceivably mediate the selective vulnerability of ATL to TDP-C. Future work will focus on characterizing the distinctive features of the abnormal TDP-C neurites, the mechanisms of neurotoxicity, initial cellular targets within the ATL, trajectory of spread, and the nature of ATL-specific markers that modulate vulnerability to TDP-C. ANN NEUROL 2023;94:1-12.
© 2023 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
CONFLICT OF INTEREST: Nothing to report.
Figures

Similar articles
-
Proteomics of the temporal cortex in semantic dementia reveals brain-region specific molecular pathology and regulation of the TDP-43-ANXA11 interactome.Acta Neuropathol Commun. 2025 Jul 25;13(1):162. doi: 10.1186/s40478-025-02077-x. Acta Neuropathol Commun. 2025. PMID: 40713859 Free PMC article.
-
Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report.Brain. 2019 Jun 1;142(6):1503-1527. doi: 10.1093/brain/awz099. Brain. 2019. PMID: 31039256 Free PMC article.
-
Cytoarchitectonic gradients of laminar degeneration in behavioural variant frontotemporal dementia.Brain. 2025 Jan 7;148(1):102-118. doi: 10.1093/brain/awae263. Brain. 2025. PMID: 39119853 Free PMC article.
-
Regional cerebral blood flow single photon emission computed tomography for detection of Frontotemporal dementia in people with suspected dementia.Cochrane Database Syst Rev. 2015 Jun 23;2015(6):CD010896. doi: 10.1002/14651858.CD010896.pub2. Cochrane Database Syst Rev. 2015. PMID: 26102272 Free PMC article.
-
TDP-43 as a possible biomarker for frontotemporal lobar degeneration: a systematic review of existing antibodies.Acta Neuropathol Commun. 2015 Apr 1;3:15. doi: 10.1186/s40478-015-0195-1. Acta Neuropathol Commun. 2015. PMID: 25853864 Free PMC article.
Cited by
-
Neural mechanisms of sentence production: a volumetric study of primary progressive aphasia.Cereb Cortex. 2024 Jan 14;34(1):bhad470. doi: 10.1093/cercor/bhad470. Cereb Cortex. 2024. PMID: 38100360 Free PMC article.
-
Assessing network degeneration and phenotypic heterogeneity in genetic frontotemporal lobar degeneration by decoding FDG-PET.Neuroimage Clin. 2024;41:103559. doi: 10.1016/j.nicl.2023.103559. Epub 2023 Dec 22. Neuroimage Clin. 2024. PMID: 38147792 Free PMC article.
-
Spatial and Temporal Progression of Neurodegeneration in Confirmed and Suspected TDP-43 Type C Pathology.Imaging Neurosci (Camb). 2025;3:IMAG.a.83. doi: 10.1162/imag.a.83. Epub 2025 Jul 16. Imaging Neurosci (Camb). 2025. PMID: 40761602 Free PMC article.
-
The Media Coverage of Bruce Willis Reveals Unfamiliarity With Frontotemporal Degeneration.Innov Aging. 2023 Oct 26;7(9):igad125. doi: 10.1093/geroni/igad125. eCollection 2023. Innov Aging. 2023. PMID: 38046892 Free PMC article.
-
Noninvasive Brain Stimulation in Primary Progressive Aphasia with and Without Concomitant Speech and Language Therapy: Systematic Review and Meta-analysis.Neuropsychol Rev. 2025 Feb 1. doi: 10.1007/s11065-025-09659-5. Online ahead of print. Neuropsychol Rev. 2025. PMID: 39893271 Review.
References
-
- Jagust WJ, Davies P, Tiller-Borcich JK, Reed BR. Focal Alzheimer’s disease. Neurology. 1990;40:14–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

