Paving a way to treat spastic paraplegia 50
- PMID: 37183815
- PMCID: PMC10178831
- DOI: 10.1172/JCI170226
Paving a way to treat spastic paraplegia 50
Abstract
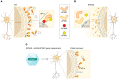
Spastic paraplegia 50 (SPG50) is a rare neurodegenerative disease caused by loss-of-function mutations in AP4M1. There are no effective treatments for SPG50 or any other type of SPG, and current treatments are limited to symptomatic management. In this issue of the JCI, Chen et al. provide promising data from preclinical studies that evaluated the efficacy and safety profiles of an AAV-mediated AP4M1 gene replacement therapy for SPG50. AAV/AP4M1 gene replacement partly rescued functional defects in SPG50 cellular and mouse models, with acceptable safety profiles in rodents and monkeys. This work represents a substantial advancement in therapeutic development of SPG50 treatments, establishing the criteria for taking AAV9/AP4M1 gene therapy to clinical trials.
Conflict of interest statement
Figures
Comment on
-
Intrathecal AAV9/AP4M1 gene therapy for hereditary spastic paraplegia 50 shows safety and efficacy in preclinical studies.J Clin Invest. 2023 May 15;133(10):e164575. doi: 10.1172/JCI164575. J Clin Invest. 2023. PMID: 36951961 Free PMC article.
References
-
- Ebrahimi-Fakhari D, et al. AP-4-Associated Hereditary Spastic Paraplegia. In: Adam MP, et al, eds. GeneReviews. University of Washington; 2018. - PubMed

