Izokibep: Preclinical development and first-in-human study of a novel IL-17A neutralizing Affibody molecule in patients with plaque psoriasis
- PMID: 37184136
- PMCID: PMC10187109
- DOI: 10.1080/19420862.2023.2209920
Izokibep: Preclinical development and first-in-human study of a novel IL-17A neutralizing Affibody molecule in patients with plaque psoriasis
Abstract
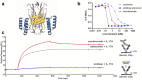
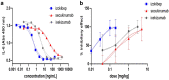
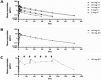
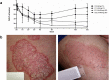
Psoriasis, an immune-mediated inflammatory disease, affects nearly 125 million people globally. The interleukin (IL)-17A homodimer is a key driver of psoriasis and other autoimmune diseases, including psoriatic arthritis, axial spondyloarthritis, hidradenitis suppurativa, and uveitis. Treatment with monoclonal antibodies (mAbs) against IL-17A provides an improvement in the Psoriasis Area and Severity Index compared to conventional systemic agents. In this study, the AffibodyⓇ technology was used to identify and optimize a novel, small, biological molecule comprising three triple helical affinity domains, izokibep (previously ABY-035), for the inhibition of IL-17A signaling. Preclinical studies show that izokibep, a small 18.6 kDa IL-17 ligand trap comprising two IL-17A-specific Affibody domains and one albumin-binding domain, selectively inhibits human IL-17A in vitro and in vivo with superior potency and efficacy relative to anti-IL-17A mAbs. A Phase 1 first-in-human study was conducted to establish the safety, pharmacokinetics, and preliminary efficacy of izokibep, when administered intravenously and subcutaneously as single doses to healthy subjects, and as single intravenous and multiple subcutaneous doses to patients with psoriasis (NCT02690142; EudraCT No: 2015-004531-13). Izokibep was well tolerated with no meaningful safety concerns identified in healthy volunteers and patients with psoriasis. Rapid efficacy was seen in all psoriasis patients after one dose which further improved in patients receiving multiple doses. A therapeutic decrease in joint pain was also observed in a single patient with concurrent psoriatic arthritis. The study suggests that izokibep has the potential to safely treat IL17A-associated diseases such as psoriasis, psoriatic arthritis, axial spondyloarthritis, hidradenitis suppurativa, and uveitis.
Keywords: ABY-035; IL-17A inhibition; first-in-human; izokibep; psoriasis.
Conflict of interest statement
SK, JF, KDB, EG, IHG, DB and FYF are currently, and LG and AW were previously employees of Affibody AB and its affiliates. ACN, APK, and PMP are employees of ACELYRIN.
Figures

References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
