An in silico FSHD muscle fiber for modeling DUX4 dynamics and predicting the impact of therapy
- PMID: 37184373
- PMCID: PMC10287159
- DOI: 10.7554/eLife.88345
An in silico FSHD muscle fiber for modeling DUX4 dynamics and predicting the impact of therapy
Abstract
Facioscapulohumeral muscular dystrophy (FSHD) is an incurable myopathy linked to the over-expression of the myotoxic transcription factor DUX4. Targeting DUX4 is the leading therapeutic approach, however, it is only detectable in 0.1-3.8% of FSHD myonuclei. How rare DUX4 drives FSHD and the optimal anti-DUX4 strategy are unclear. We combine stochastic gene expression with compartment models of cell states, building a simulation of DUX4 expression and consequences in FSHD muscle fibers. Investigating iDUX4 myoblasts, scRNAseq, and snRNAseq of FSHD muscle we estimate parameters including DUX4 mRNA degradation, transcription and translation rates, and DUX4 target gene activation rates. Our model accurately recreates the distribution of DUX4 and targets gene-positive cells seen in scRNAseq of FSHD myocytes. Importantly, we show DUX4 drives significant cell death despite expression in only 0.8% of live cells. Comparing scRNAseq of unfused FSHD myocytes to snRNAseq of fused FSHD myonuclei, we find evidence of DUX4 protein syncytial diffusion and estimate its rate via genetic algorithms. We package our model into freely available tools, to rapidly investigate the consequences of anti-DUX4 therapy.
Keywords: DUX4; FSHD; cell biology; cellular automaton; computational biology; facioscapulohumeral muscular dystrophy; human; muscle; stochastic gene expression; systems biology.
© 2023, Cowley, Pruller et al.
Conflict of interest statement
MC, JP, MG, PZ, CB No competing interests declared
Figures

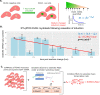




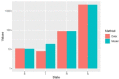
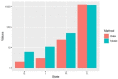
Update of
References
-
- Banerji CRS, Panamarova M, Pruller J, Figeac N, Hebaishi H, Fidanis E, Saxena A, Contet J, Sacconi S, Severini S, Zammit PS. Dynamic Transcriptomic analysis reveals suppression of Pgc1Α/ERRα drives perturbed Myogenesis in Facioscapulohumeral muscular dystrophy. Human Molecular Genetics. 2019;28:1244–1259. doi: 10.1093/hmg/ddy405. - DOI - PMC - PubMed
-
- Banerji CRS, Cammish P, Evangelista T, Zammit PS, Straub V, Marini-Bettolo C. Facioscapulohumeral muscular dystrophy 1 patients participating in the UK FSHD Registry can be Subdivided into 4 patterns of self-reported symptoms. Neuromuscular Disorders. 2020a;30:315–328. doi: 10.1016/j.nmd.2020.03.001. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

