Safety, tolerability, and efficacy of maralixibat in adults with primary sclerosing cholangitis: Open-label pilot study
- PMID: 37184523
- PMCID: PMC10187837
- DOI: 10.1097/HC9.0000000000000153
Safety, tolerability, and efficacy of maralixibat in adults with primary sclerosing cholangitis: Open-label pilot study
Abstract
Background: Primary sclerosing cholangitis (PSC) is frequently associated with pruritus, which significantly impairs quality of life. Maralixibat is a selective ileal bile acid transporter (IBAT) inhibitor that lowers circulating bile acid (BA) levels and reduces pruritus in cholestatic liver diseases. This is the first proof-of-concept study of IBAT inhibition in PSC.
Methods: This open-label study evaluated the safety and tolerability of maralixibat ≤10 mg/d for 14 weeks in adults with PSC. Measures of pruritus, biomarkers of BA synthesis, cholestasis, and liver function were also assessed.
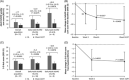
Results: Of 27 enrolled participants, 85.2% completed treatment. Gastrointestinal treatment-emergent adverse events (TEAEs) occurred in 81.5%, with diarrhea in 51.9%. TEAEs were mostly mild or moderate (63.0%); 1 serious TEAE (cholangitis) was considered treatment related. Mean serum BA (sBA) levels decreased by 16.7% (-14.84 µmol/L; 95% CI, -27.25 to -2.43; p = 0.0043) by week 14/early termination (ET). In participants with baseline sBA levels above normal (n = 18), mean sBA decreased by 40.0% (-22.3 µmol/L, 95% CI, -40.38 to -4.3; p = 0.004) by week 14/ET. Liver enzyme elevations were not significant; however, increases of unknown clinical significance in conjugated bilirubin levels were observed. ItchRO weekly sum scores decreased from baseline to week 14/ET by 8.4% (p = 0.0495), by 12.6% (p = 0.0275) in 18 participants with pruritus at baseline, and by 70% (p = 0.0078) in 8 participants with ItchRO daily average score ≥3 at baseline.
Conclusions: Maralixibat was associated with reduced sBA levels in adults with PSC. In participants with more severe baseline pruritus, pruritus improved significantly from baseline. TEAEs were mostly gastrointestinal related. These results support further investigation of IBAT inhibitors for adults with PSC-associated pruritus. ClinicalTrials.gov: NCT02061540.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
Christopher L. Bowlus has received payments through their institution from Mirum Pharmaceuticals, Inc.; received grants from CymaBay, BMS, GSK, Intercept, Hanmi, TARGET, Pliant, Eli Lilly, GENFIT, Novartis, Takeda, Arena Pharmaceuticals, and Calliditas; and served as a consultant for CymaBay, GSK, Eli Lilly, Shire, and Mirum Pharmaceuticals Inc. Cynthia A. Moylan has received research grants from Exact Sciences and GSK. Paul J. Pockros has served as an advisor for Intercept, Gilead, and AbbVie; served as a speaker for Intercept; received research grants from Intercept; and received unrestricted grants from Gilead and AbbVie. Alejandro Dorenbaum was a prior stock owner (fully divested) of Mirum Pharmaceuticals Inc. Ciara Kennedy is a stock holder and company founder of Mirum Pharmaceuticals Inc. Thomas Jaecklin owns stocks and has intellectual property rights in Mirum Pharmaceuticals Inc. and owns stocks in and is employed by Galapagos. Andrew McKibben owns stocks in and is employed by Mirum Pharmaceuticals Inc. Elaine Chien owns stocks in and is employed by Mirum Pharmaceuticals Inc. Marshall Baek owns stocks in and is employed by Mirum Pharmaceuticals Inc. Pamela Vig owns stocks in and is employed by Mirum Pharmaceuticals Inc. Cynthia Levy has received research grants through their institution from Calliditas, Cara Therapeutics, CymaBay, GENFIT, Gilead, GSK, Intercept, Novartis, HighTide, Zydus, Mirum Pharmaceuticals Inc., Escient, Pliant, and Target PharmaSolutions; has served as a consultant for CymaBay, GENFIT, DISC, GSK, Ipsen, Pliant, Mirum Pharmaceuticals Inc., Calliditas, and Intercept; serves as associate editor for
Figures

References
-
- Baluyut AR, Sherman S, Lehman GA, Hoen H, Chalasani N. Impact of endoscopic therapy on the survival of patients with primary sclerosing cholangitis. Gastrointest Endosc. 2001;53:308–12. - PubMed

