Identification of an anergic BND cell-derived activated B cell population (BND2) in young-onset type 1 diabetes patients
- PMID: 37184563
- PMCID: PMC10192302
- DOI: 10.1084/jem.20221604
Identification of an anergic BND cell-derived activated B cell population (BND2) in young-onset type 1 diabetes patients
Abstract
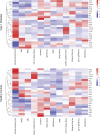
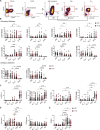
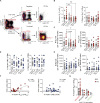
Recent evidence suggests a role for B cells in the pathogenesis of young-onset type 1 diabetes (T1D), wherein rapid progression occurs. However, little is known regarding the specificity, phenotype, and function of B cells in young-onset T1D. We performed a cross-sectional analysis comparing insulin-reactive to tetanus-reactive B cells in the blood of T1D and controls using mass cytometry. Unsupervised clustering revealed the existence of a highly activated B cell subset we term BND2 that falls within the previously defined anergic BND subset. We found a specific increase in the frequency of insulin-reactive BND2 cells in the blood of young-onset T1D donors, which was further enriched in the pancreatic lymph nodes of T1D donors. The frequency of insulin-binding BND2 cells correlated with anti-insulin autoantibody levels. We demonstrate BND2 cells are pre-plasma cells and can likely act as APCs to T cells. These findings identify an antigen-specific B cell subset that may play a role in the rapid progression of young-onset T1D.
© 2023 Stensland et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures


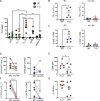
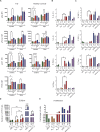
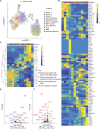
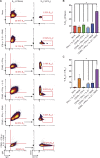

References
-
- Bingley, P.J., Boulware D.C., Krischer J.P., Type G., and Type 1 Diabetes TrialNet Study Group . 2016. The implications of autoantibodies to a single islet antigen in relatives with normal glucose tolerance: Development of other autoantibodies and progression to type 1 diabetes. Diabetologia. 59:542–549. 10.1007/s00125-015-3830-2 - DOI - PMC - PubMed
-
- Cowan, G.J.M., Miles K., Capitani L., Giguere S.S.B., Johnsson H., Goodyear C., McInnes I.B., Breusch S., Gray D., and Gray M.. 2020. In human autoimmunity, a substantial component of the B cell repertoire consists of polyclonal, barely mutated IgG+ve B cells. Front. Immunol. 11:395. 10.3389/fimmu.2020.00395 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

