Efficacy of a Vibrating Crib Mattress to Reduce Pharmacologic Treatment in Opioid-Exposed Newborns: A Randomized Clinical Trial
- PMID: 37184872
- PMCID: PMC10186209
- DOI: 10.1001/jamapediatrics.2023.1077
Efficacy of a Vibrating Crib Mattress to Reduce Pharmacologic Treatment in Opioid-Exposed Newborns: A Randomized Clinical Trial
Abstract
Importance: Pharmacologic agents are often used to treat newborns with prenatal opioid exposure (POE) despite known adverse effects on neurodevelopment. Alternative nonpharmacological interventions are needed.
Objective: To examine efficacy of a vibrating crib mattress for treating newborns with POE.
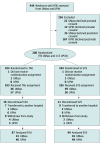
Design, setting, and participants: In this dual-site randomized clinical trial, 208 term newborns with POE, enrolled from March 9, 2017, to March 10, 2020, were studied at their bedside throughout hospitalization.
Interventions: Half the cohort received treatment as usual (TAU) and half received standard care plus low-level stochastic (random) vibrotactile stimulation (SVS) using a uniquely constructed crib mattress with a 3-hour on-off cycle. Study initiated in the newborn unit where newborns were randomized to TAU or SVS within 48 hours of birth. All infants whose symptoms met clinical criteria for pharmacologic treatment received morphine in the neonatal intensive care unit per standard care.
Main outcomes and measures: The a priori primary outcomes analyzed were pharmacotherapy (administration of morphine treatment [AMT], first-line medication at both study sites [number of infants treated], and cumulative morphine dose) and hospital length of stay. Intention-to-treat analysis was conducted.
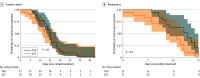
Results: Analyses were performed on 181 newborns who completed hospitalization at the study sites (mean [SD] gestational age, 39.0 [1.2] weeks; mean [SD] birth weight, 3076 (489) g; 100 [55.2%] were female). Of the 181 analyzed infants, 121 (66.9%) were discharged without medication and 60 (33.1%) were transferred to the NICU for morphine treatment (31 [51.7%] TAU and 29 [48.3%] SVS). Treatment rate was not significantly different in the 2 groups: 35.6% (31 of 87 infants who received TAU) and 30.9% (29 of 94 infants who received SVS) (P = .60). Adjusting for site, sex, birth weight, opioid exposure, and feed type, infant duration on the vibrating mattress in the newborn unit was associated with reduction in AMT (adjusted odds ratio, 0.88 hours per day; 95% CI, 0.81-0.93 hours per day). This translated to a 50% relative reduction in AMT for infants who received SVS on average 6 hours per day. Among 32 infants transferred to the neonatal intensive care unit for morphine treatment who completed treatment within 3 weeks, those assigned to SVS finished treatment nearly twice as fast (hazard ratio, 1.96; 95% CI, 1.01-3.81), resulting in 3.18 fewer treatment days (95% CI, -0.47 to -0.04 days) and receiving a mean 1.76 mg/kg less morphine (95% CI, -3.02 to -0.50 mg/kg) than the TAU cohort. No effects of condition were observed among infants treated for more than 3 weeks (n = 28).
Conclusions and relevance: The findings of this clinical trial suggest that SVS may serve as a complementary nonpharmacologic intervention for newborns with POE. Reducing pharmacotherapy with SVS has implications for reduced hospitalization stays and costs, and possibly improved infant outcomes given the known adverse effects of morphine on neurodevelopment.
Trial registration: ClinicalTrials.gov Identifier: NCT02801331.
Conflict of interest statement
Figures
References
-
- Velez ML, Jordan C, Jansson LM. Reconceptualizing non-pharmacologic approaches to neonatal abstinence syndrome (NAS) and neonatal opioid withdrawal syndrome (NOWS): a theoretical and evidence-based approach—part II: the clinical application of nonpharmacologic care for NAS/NOWS. Neurotoxicol Teratol. 2021;88:107032. doi: 10.1016/j.ntt.2021.107032 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

