Millisecond cryo-trapping by the spitrobot crystal plunger simplifies time-resolved crystallography
- PMID: 37185266
- PMCID: PMC10130016
- DOI: 10.1038/s41467-023-37834-w
Millisecond cryo-trapping by the spitrobot crystal plunger simplifies time-resolved crystallography
Abstract
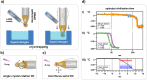
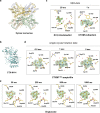
We introduce the spitrobot, a protein crystal plunger, enabling reaction quenching via cryo-trapping with a time-resolution in the millisecond range. Protein crystals are mounted on canonical micromeshes on an electropneumatic piston, where the crystals are kept in a humidity and temperature-controlled environment, then reactions are initiated via the liquid application method (LAMA) and plunging into liquid nitrogen is initiated after an electronically set delay time to cryo-trap intermediate states. High-magnification images are automatically recorded before and after droplet deposition, prior to plunging. The SPINE-standard sample holder is directly plunged into a storage puck, enabling compatibility with high-throughput infrastructure. Here we demonstrate binding of glucose and 2,3-butanediol in microcrystals of xylose isomerase, and of avibactam and ampicillin in microcrystals of the extended spectrum beta-lactamase CTX-M-14. We also trap reaction intermediates and conformational changes in macroscopic crystals of tryptophan synthase to demonstrate that the spitrobot enables insight into catalytic events.
© 2023. The Author(s).
Conflict of interest statement
On 10 March 2022, a patent application has been filed under the number EP22161384, for the Max-Planck Society and to F.T., E.C.S., H.S., P.M., M.K. under the following title: “VERFAHREN UND VORRICHTUNG ZUM BEREITSTELLEN VON BIOLOGISCHEN PROBEN IN EINEM VITRIFIZIERTEN ZUSTAND FÜR STATISCHE UND ZEITAUFGELÖSTE STRUKTURUNTERSUCHUNGEN MIT HILFE VON ELEKTRONEN- ODER RÖNTGENQUELLEN”. The remaining authors declare no competing interests.
Figures

References
-
- Bourgeois D, Weik M. Kinetic protein crystallography: a tool to watch proteins in action. Crystallogr. Rev. 2009;15:87–118. doi: 10.1080/08893110802604868. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

