A Targeted, Low-Throughput Compound Screen in a Drosophila Model of Neurofibromatosis Type 1 Identifies Simvastatin and BMS-204352 as Potential Therapies for Autism Spectrum Disorder (ASD)
- PMID: 37185294
- PMCID: PMC10198605
- DOI: 10.1523/ENEURO.0461-22.2023
A Targeted, Low-Throughput Compound Screen in a Drosophila Model of Neurofibromatosis Type 1 Identifies Simvastatin and BMS-204352 as Potential Therapies for Autism Spectrum Disorder (ASD)
Abstract
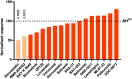
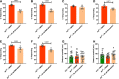
Autism spectrum disorder (ASD) is a common neurodevelopmental condition for which there are no pharmacological therapies that effectively target its core symptomatology. Animal models of syndromic forms of ASD, such as neurofibromatosis type 1, may be of use in screening for such treatments. Drosophila larvae lacking Nf1 expression exhibit tactile hypersensitivity following mechanical stimulation, proposed to mirror the sensory sensitivity issues comprising part of the ASD diagnostic criteria. Such behavior is associated with synaptic dysfunction at the neuromuscular junction (NMJ). Both phenotypes may thus provide tractable outputs with which to screen for potential ASD therapies. In this study, we demonstrate that, while loss of Nf1 expression within the embryo is sufficient to impair NMJ synaptic transmission in the larva, constitutive Nf1 knock-down is required to induce tactile hypersensitivity, suggesting that a compound must be administered throughout development to rescue this behavior. With such a feeding regime, we identify two compounds from a targeted, low-throughput screen that significantly and consistently reduce, but do not fully rescue, tactile hypersensitivity in Nf1P1 larvae. These are the HMG CoA-reductase inhibitor simvastatin, and the BKCa channel activator BMS-204352. At the NMJ, both compounds induce a significant reduction in the enhanced spontaneous transmission frequency of Nf1P1 larvae, though again not to the level of vehicle-treated controls. However, both compounds fully rescue the increased quantal size of Nf1P1 mutants, with simvastatin also fully rescuing their reduced quantal content. Thus, the further study of both compounds as potential ASD interventions is warranted.
Keywords: Drosophila; Nf1; autism spectrum disorder; drug screening; neurofibromatosis type 1.
Copyright © 2023 Dyson et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5, Ed 5. Washington, DC: American Psychiatric Association.
-
- Ashburner M, Golic K, Hawley R (2005) Temperature and development. In: Drosophila: a laboratory handbook, pp 161–163. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
