Scalable continuous-flow electroporation platform enabling T cell transfection for cellular therapy manufacturing
- PMID: 37185305
- PMCID: PMC10133335
- DOI: 10.1038/s41598-023-33941-2
Scalable continuous-flow electroporation platform enabling T cell transfection for cellular therapy manufacturing
Abstract
Viral vectors represent a bottleneck in the manufacturing of cellular therapies. Electroporation has emerged as an approach for non-viral transfection of primary cells, but standard cuvette-based approaches suffer from low throughput, difficult optimization, and incompatibility with large-scale cell manufacturing. Here, we present a novel electroporation platform capable of rapid and reproducible electroporation that can efficiently transfect small volumes of cells for research and process optimization and scale to volumes required for applications in cellular therapy. We demonstrate delivery of plasmid DNA and mRNA to primary human T cells with high efficiency and viability, such as > 95% transfection efficiency for mRNA delivery with < 2% loss of cell viability compared to control cells. We present methods for scaling delivery that achieve an experimental throughput of 256 million cells/min. Finally, we demonstrate a therapeutically relevant modification of primary T cells using CRISPR/Cas9 to knockdown T cell receptor (TCR) expression. This study displays the capabilities of our system to address unmet needs for efficient, non-viral engineering of T cells for cell manufacturing.
© 2023. The Author(s).
Conflict of interest statement
JAV, TNC, and HGC are listed as inventors on patent applications related to the technology presented and JAV, TNC, SLL, and HGC have a financial interest in CyteQuest.
Figures

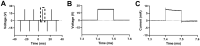
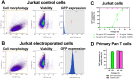
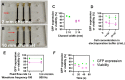



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

