Anti-cancer pro-inflammatory effects of an IgE antibody targeting the melanoma-associated antigen chondroitin sulfate proteoglycan 4
- PMID: 37185332
- PMCID: PMC10130092
- DOI: 10.1038/s41467-023-37811-3
Anti-cancer pro-inflammatory effects of an IgE antibody targeting the melanoma-associated antigen chondroitin sulfate proteoglycan 4
Abstract
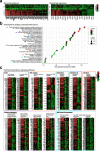
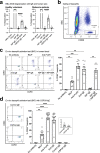
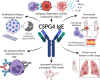
Outcomes for half of patients with melanoma remain poor despite standard-of-care checkpoint inhibitor therapies. The prevalence of the melanoma-associated antigen chondroitin sulfate proteoglycan 4 (CSPG4) expression is ~70%, therefore effective immunotherapies directed at CSPG4 could benefit many patients. Since IgE exerts potent immune-activating functions in tissues, we engineer a monoclonal IgE antibody with human constant domains recognizing CSPG4 to target melanoma. CSPG4 IgE binds to human melanomas including metastases, mediates tumoricidal antibody-dependent cellular cytotoxicity and stimulates human IgE Fc-receptor-expressing monocytes towards pro-inflammatory phenotypes. IgE demonstrates anti-tumor activity in human melanoma xenograft models engrafted with human effector cells and is associated with enhanced macrophage infiltration, enriched monocyte and macrophage gene signatures and pro-inflammatory signaling pathways in the tumor microenvironment. IgE prolongs the survival of patient-derived xenograft-bearing mice reconstituted with autologous immune cells. No ex vivo activation of basophils in patient blood is measured in the presence of CSPG4 IgE. Our findings support a promising IgE-based immunotherapy for melanoma.
© 2023. The Author(s).
Conflict of interest statement
J.S. and S.N.K. are founders and shareholders of Epsilogen Ltd. S.N.K., J.S., D.H.J., G.P., and H.J.B. declare patents on antibodies for cancer. J.C., M.G., J.L-A, H.S.S, and H.J.B have been employed through a fund provided by Epsilogen Ltd. P.K. has conducted advisory work for Janssen and is stockholder in Abbvie. J.D. has acted as a consultant for AstraZeneca, Jubilant, Theras, BridgeBio and Vividion, and has funded research agreements with BMS and Revolution Medicines. All other authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

