Systematic Literature Review (SLR) and Network Meta-Analysis (NMA) of First-Line Therapies (1L) for Locally Advanced/Metastatic Urothelial Carcinoma (la/mUC)
- PMID: 37185390
- PMCID: PMC10136539
- DOI: 10.3390/curroncol30040277
Systematic Literature Review (SLR) and Network Meta-Analysis (NMA) of First-Line Therapies (1L) for Locally Advanced/Metastatic Urothelial Carcinoma (la/mUC)
Abstract
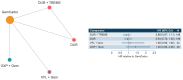
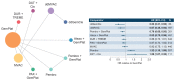
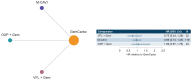
To compare efficacy outcomes for all approved and investigational first-line (1L) treatment regimens for locally advanced or metastatic urothelial carcinoma (la/mUC) with standard of care (SOC), a network meta-analysis (NMA) was conducted. A systematic literature review (SLR) identified phase 2 and 3 randomized trials investigating 1L treatment regimens in la/mUC published January 2001-September 2021. Three networks were formed based on cisplatin (cis) eligibility: cis-eligible/mixed (cis-eligible patients and mixed populations of cis-eligible/ineligible patients), cis-ineligible (strict; exclusively cis-ineligible patients), and cis-ineligible (wide; including studies with investigator's choice of carbo). Analyses examined comparative efficacy by hazard ratio (HR) for overall survival (OS), and progression-free survival (PFS), and odds ratio (OR) for overall response rate (ORR), with 1L regimens vs. SOC. SOC was gemcitabine + cis (GemCis) or carboplatin (GemCarbo), cis-eligible/mixed network, and GemCarbo cis-ineligible networks. Of 1906 SLR identified citations, 55 trials were selected for data extraction. The NMA comprised 11, 6, and 8 studies in the cis-eligible/mixed, cis-ineligible (strict), cis-ineligible (wide) networks, respectively. In a meta-analysis of SOC control arms, median (95% CI) overall survival (OS) in months varied by network: 13.19 (12.43, 13.95) cis-eligible/mixed, 11.96 (10.43, 13.48) cis-ineligible (wide), and 9.74 (6.71, 12.76) cis-ineligible (strict). Most differences in OS, PFS, and ORR with treatment regimens across treatment networks were not statistically significant compared with SOC. Outcomes with current 1L regimens remain poor, and few significant improvements over SOC have been made, despite inclusion of recent clinical trial data, highlighting an unmet need in the la/mUC patient population.
Keywords: bladder cancer; network meta-analysis; oncology; overall survival; standard of care; systematic literature review.
Conflict of interest statement
This study was sponsored by Seagen Inc. P.W., C.L.D., J.S.L. and Z.H. are employees of, and hold stock/stock options in, Seagen Inc. L.B. is an employee of Curta Inc. C.M. and E.L. are employees of Astellas Pharma Global Development, Inc. C.M. also holds stock/stock options in J&J and Merck. L.B., S.D.R., S.D.S. and B.D. received support from Seagen for this research. S.D.R. has previously performed paid consulting work for AstraZeneca, Bayer, Biovica, Flatiron Health, Genentech, GRAIL, Merck, and Seagen Inc.; has received institutional research funding from Bayer and Genentech/Roche; and travel expenses from Bayer. Curta Inc. and Astellas Pharma Global Development, Inc. received funding from Seagen Inc. for this research.
Figures



References
-
- National Institutes of Health. National Cancer Institute. Surveillance Epidemiology and End Results Program Cancer Stat Facts: Bladder Cancer. [(accessed on 22 July 2022)]; Available online: https://seer.cancer.gov/statfacts/html/urinb.html.
-
- National Comprehensive Cancer Network NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Bladder Cancer. 2022. [(accessed on 9 August 2022)]. Available online: nccn.org.
-
- Powles T., Bellmunt J., Comperat E., De Santis M., Huddart R., Loriot Y., Necchi A., Valderrama B.P., Ravaud A., Shariat S.F., et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2021;33:244–258. doi: 10.1016/j.annonc.2021.11.012. - DOI - PubMed
-
- Galsky M.D., Hahn N.M., Rosenberg J., Sonpavde G., Hutson T., Oh W.K., Dreicer R., Vogelzang N., Sternberg C.N., Bajorin D.F., et al. Treatment of Patients with Metastatic Urothelial Cancer “Unfit” for Cisplatin-Based Chemotherapy. J. Clin. Oncol. 2011;29:2432–2438. doi: 10.1200/JCO.2011.34.8433. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

