Impact of the Extremities Positioning on the Set-Up Reproducibility for the Total Marrow Irradiation Treatment
- PMID: 37185422
- PMCID: PMC10136565
- DOI: 10.3390/curroncol30040309
Impact of the Extremities Positioning on the Set-Up Reproducibility for the Total Marrow Irradiation Treatment
Abstract
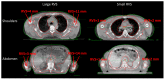
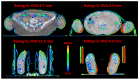
Total marrow (lymph node) irradiation (TMI/TMLI) delivery requires more time than standard radiotherapy treatments. The patient's extremities, through the joints, can experience large movements. The reproducibility of TMI/TMLI patients' extremities was evaluated to find the best positioning and reduce unwanted movements. Eighty TMI/TMLI patients were selected (2013-2022). During treatment, a cone-beam computed tomography (CBCT) was performed for each isocenter to reposition the patient. CBCT-CT pairs were evaluated considering: (i) online vector shift (OVS) that matched the two series; (ii) residual vector shift (RVS) to reposition the patient's extremities; (iii) qualitative agreement (range 1-5). Patients were subdivided into (i) arms either leaning on the frame or above the body; (ii) with or without a personal cushion for foot positioning. The Mann-Whitney test was considered (p < 0.05 significant). Six-hundred-twenty-nine CBCTs were analyzed. The median OVS was 4.0 mm, with only 1.6% of cases ranked < 3, and 24% of RVS > 10 mm. Arms leaning on the frame had significantly smaller RVS than above the body (median: 8.0 mm/6.0 mm, p < 0.05). Using a personal cushion for the feet significantly improved the RVS than without cushions (median: 8.5 mm/1.8 mm, p < 0.01). The role and experience of the radiotherapy team are fundamental to optimizing the TMI/TMLI patient setup.
Keywords: patient positioning; radiotherapy (RT); reproducibility; total marrow irradiation (TMI); total marrow lymph node irradiation (TMLI); volumetric modulated arc therapy (VMAT).
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Peters C., Dalle J.-H., Locatelli F., Poetschger U., Sedlacek P., Buechner J., Shaw P.J., Staciuk R., Ifversen M., Pichler H., et al. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. JCO. 2021;39:295–307. doi: 10.1200/JCO.20.02529. - DOI - PMC - PubMed
-
- Blaise D., Maraninchi D., Michallet M., Reiffers J., Jouet J.P., Milpied N., Devergie A., Attal M., Sotto J.J., Kuentz M., et al. Long-Term Follow-up of a Randomized Trial Comparing the Combination of Cyclophosphamide with Total Body Irradiation or Busulfan as Conditioning Regimen for Patients Receiving HLA-Identical Marrow Grafts for Acute Myeloblastic Leukemia in First Complete Remission. Blood. 2001;97:3669–3671. doi: 10.1182/blood.V97.11.3669. - DOI - PubMed
-
- Bunin N., Aplenc R., Kamani N., Shaw K., Cnaan A., Simms S. Randomized Trial of Busulfan vs Total Body Irradiation Containing Conditioning Regimens for Children with Acute Lymphoblastic Leukemia: A Pediatric Blood and Marrow Transplant Consortium Study. Bone Marrow Transplant. 2003;32:543–548. doi: 10.1038/sj.bmt.1704198. - DOI - PubMed
-
- Sieker K., Fleischmann M., Trommel M., Ramm U., Licher J., Bug G., Martin H., Serve H., Rödel C., Balermpas P. Twenty Years of Experience of a Tertiary Cancer Center in Total Body Irradiation with Focus on Oncological Outcome and Secondary Malignancies. Strahlenther. Onkol. 2022;198:547–557. doi: 10.1007/s00066-022-01914-5. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

