The long non-coding RNA keratin-7 antisense acts as a new tumor suppressor to inhibit tumorigenesis and enhance apoptosis in lung and breast cancers
- PMID: 37185462
- PMCID: PMC10130017
- DOI: 10.1038/s41419-023-05802-3
The long non-coding RNA keratin-7 antisense acts as a new tumor suppressor to inhibit tumorigenesis and enhance apoptosis in lung and breast cancers
Abstract
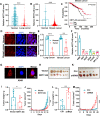
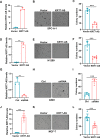
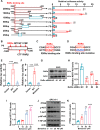
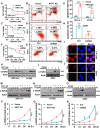
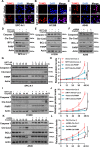
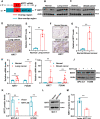
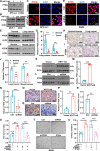
Expression of the long non-coding RNA (lncRNA) keratin-7 antisense (KRT7-AS) is downregulated in various types of cancer; however, the impact of KRT7-AS deficiency on tumorigenesis and apoptosis is enigmatic. We aim to explore the influence of KRT7-AS in carcinogenesis and apoptosis. We found that KRT7-AS was deficient in breast and lung cancers, and low levels of KRT7-AS were a poor prognostic factor in breast cancer. Cellular studies showed that silencing of KRT7-AS in lung cancer cells increased oncogenic Keratin-7 levels and enhanced tumorigenesis, but diminished cancer apoptosis of the cancer cells; by contrast, overexpression of KRT7-AS inhibited lung cancer cell tumorigenesis. Additionally, KRT7-AS sensitized cancer cells to the anti-cancer drug cisplatin, consequently enhancing cancer cell apoptosis. In vivo, KRT7-AS overexpression significantly suppressed tumor growth in xenograft mice, while silencing of KRT7-AS promoted tumor growth. Mechanistically, KRT7-AS reduced the levels of oncogenic Keratin-7 and significantly elevated amounts of the key tumor suppressor PTEN in cancer cells through directly binding to PTEN protein via its core nucleic acid motif GGCAAUGGCGG. This inhibited the ubiquitination-proteasomal degradation of PTEN protein, therefore elevating PTEN levels in cancer cells. We also found that KRT7-AS gene transcription was driven by the transcription factor RXRα; intriguingly, the small molecule berberine enhanced KRT7-AS expression, reduced tumorigenesis, and promoted apoptosis of cancer cells. Collectively, KRT7-AS functions as a new tumor suppressor and an apoptosis enhancer in lung and breast cancers, and we unraveled that the RXRα-KRT7-AS-PTEN signaling axis controls carcinogenesis and apoptosis. Our findings highlight a tumor suppressive role of endogenous KRT7-AS in cancers and an important effect the RXRα-KRT7-AS-PTEN axis on control of cancer cell tumorigenesis and apoptosis, and offer a new platform for developing novel therapeutics against cancers.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Long non-coding antisense RNA KRT7-AS is activated in gastric cancers and supports cancer cell progression by increasing KRT7 expression.Oncogene. 2016 Sep 15;35(37):4927-36. doi: 10.1038/onc.2016.25. Epub 2016 Feb 15. Oncogene. 2016. PMID: 26876208 Free PMC article.
-
N6 -Methyladenosine Regulates mRNA Stability and Translation Efficiency of KRT7 to Promote Breast Cancer Lung Metastasis.Cancer Res. 2021 Jun 1;81(11):2847-2860. doi: 10.1158/0008-5472.CAN-20-3779. Epub 2021 Apr 1. Cancer Res. 2021. PMID: 33795252
-
Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT7.Gut Microbes. 2020 May 3;11(3):511-525. doi: 10.1080/19490976.2019.1695494. Epub 2020 Jan 7. Gut Microbes. 2020. PMID: 31910722 Free PMC article.
-
Small in Size, but Large in Action: microRNAs as Potential Modulators of PTEN in Breast and Lung Cancers.Biomolecules. 2021 Feb 18;11(2):304. doi: 10.3390/biom11020304. Biomolecules. 2021. PMID: 33670518 Free PMC article. Review.
-
PVT1 lncRNA in lung cancer: A key player in tumorigenesis and therapeutic opportunities.Pathol Res Pract. 2024 Jan;253:155019. doi: 10.1016/j.prp.2023.155019. Epub 2023 Dec 7. Pathol Res Pract. 2024. PMID: 38091883 Review.
Cited by
-
Pharmacokinetics, Tissue Distribution and Excretion of Demethyleneberberine, a Metabolite of Berberine, in Rats and Mice.Molecules. 2023 Nov 23;28(23):7725. doi: 10.3390/molecules28237725. Molecules. 2023. PMID: 38067456 Free PMC article.
-
Emerging insights into keratin 7 roles in tumor progression and metastasis of cancers.Front Oncol. 2024 Jan 8;13:1243871. doi: 10.3389/fonc.2023.1243871. eCollection 2023. Front Oncol. 2024. PMID: 38260844 Free PMC article. Review.
-
A Comprehensive Review of Protein Biomarkers for Invasive Lung Cancer.Curr Oncol. 2024 Aug 23;31(9):4818-4854. doi: 10.3390/curroncol31090360. Curr Oncol. 2024. PMID: 39329988 Free PMC article. Review.
-
Functions, mechanisms, and clinical applications of lncRNA LINC00857 in cancer pathogenesis.Hum Cell. 2023 Sep;36(5):1656-1671. doi: 10.1007/s13577-023-00936-0. Epub 2023 Jun 28. Hum Cell. 2023. PMID: 37378889 Review.
-
Intermediate Filaments in Breast Cancer Progression, and Potential Biomarker for Cancer Therapy: A Narrative Review.Breast Cancer (Dove Med Press). 2024 Oct 14;16:689-704. doi: 10.2147/BCTT.S489953. eCollection 2024. Breast Cancer (Dove Med Press). 2024. PMID: 39430570 Free PMC article. Review.
References
-
- Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

