Bone marrow endosteal stem cells dictate active osteogenesis and aggressive tumorigenesis
- PMID: 37185464
- PMCID: PMC10130060
- DOI: 10.1038/s41467-023-38034-2
Bone marrow endosteal stem cells dictate active osteogenesis and aggressive tumorigenesis
Abstract
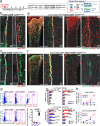
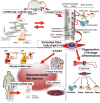
The bone marrow contains various populations of skeletal stem cells (SSCs) in the stromal compartment, which are important regulators of bone formation. It is well-described that leptin receptor (LepR)+ perivascular stromal cells provide a major source of bone-forming osteoblasts in adult and aged bone marrow. However, the identity of SSCs in young bone marrow and how they coordinate active bone formation remains unclear. Here we show that bone marrow endosteal SSCs are defined by fibroblast growth factor receptor 3 (Fgfr3) and osteoblast-chondrocyte transitional (OCT) identities with some characteristics of bone osteoblasts and chondrocytes. These Fgfr3-creER-marked endosteal stromal cells contribute to a stem cell fraction in young stages, which is later replaced by Lepr-cre-marked stromal cells in adult stages. Further, Fgfr3+ endosteal stromal cells give rise to aggressive osteosarcoma-like lesions upon loss of p53 tumor suppressor through unregulated self-renewal and aberrant osteogenic fates. Therefore, Fgfr3+ endosteal SSCs are abundant in young bone marrow and provide a robust source of osteoblasts, contributing to both normal and aberrant osteogenesis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

