Optical Detection of Cancer Cells Using Lab-on-a-Chip
- PMID: 37185514
- PMCID: PMC10136345
- DOI: 10.3390/bios13040439
Optical Detection of Cancer Cells Using Lab-on-a-Chip
Abstract
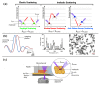
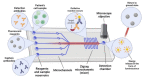
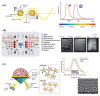
The global need for accurate and efficient cancer cell detection in biomedicine and clinical diagnosis has driven extensive research and technological development in the field. Precision, high-throughput, non-invasive separation, detection, and classification of individual cells are critical requirements for successful technology. Lab-on-a-chip devices offer enormous potential for solving biological and medical problems and have become a priority research area for microanalysis and manipulating cells. This paper reviews recent developments in the detection of cancer cells using the microfluidics-based lab-on-a-chip method, focusing on describing and explaining techniques that use optical phenomena and a plethora of probes for sensing, amplification, and immobilization. The paper describes how optics are applied in each experimental method, highlighting their advantages and disadvantages. The discussion includes a summary of current challenges and prospects for cancer diagnosis.
Keywords: cancer; chemiluminescence; fluorescence; lab-on-a-chip; optical detection; surface plasmon resonance SPR; surface-enhanced Raman scattering SERS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

